Effect of tocilizumab versus standard of care in adults hospitalized with moderate-severe COVID-19 pneumonia
- PMID: 35531306
- PMCID: PMC9063125
- DOI: 10.1016/j.medcle.2021.03.036
Effect of tocilizumab versus standard of care in adults hospitalized with moderate-severe COVID-19 pneumonia
Abstract
Introduction and objectives: Tocilizumab is an interleukin-6 receptor-blocking agent proposed for the treatment of severe COVID-19; however, limited data are available on their efficacy. The aim of this study was to assess the effect of tocilizumab on the outcomes of patients with COVID-19 pneumonia by using propensity-score-matching (PSM) analysis.
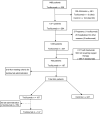
Methods: A retrospective observational analysis of hospitalized COVID-19 adult patients admitted to the Vall d'Hebron Hospital was performed between March and April 2020. We used the logistic regression to analyze the effect of tocilizumab on mortality, as main outcome, and PSM analysis to further validate their effect. Secondary outcomes were length-of-stay (LOS) and intensive-care-unit (ICU) stay. Same outcomes were also assessed for early tocilizumab administration, within 72 h after admission. Patients were selected by matching their individual propensity for receiving therapy with tocilizumab, conditional on their demographic and clinical variables.
Results: A total of 544 COVID-19 patients were included, 197 (36.2%) were treated with tocilizumab of whom 147 were treated within the first 72 h after admission; and 347 were included in the control group. After PSM analyses, the results showed no association between tocilizumab use and overall mortality (OR = 1.03, 95%CI: 0.63-1.68). However, shorter ICU-stay in the tocilizumab group was found compared to the control group (Coefficient -4.27 95%CI: -6.63 to -1.92). Similar results were found in the early tocilizumab cohort.
Conclusions: The administration of tocilizumab in patients with moderate to severe COVID-19 did not reduce the risk of mortality in our cohort of patients, regardless of the time of administration.
Introducción y objetivos: El tocilizumab es un agente bloqueador del receptor de la interleucina 6 propuesto para el tratamiento de la COVID-19 grave; sin embargo, se dispone de datos limitados sobre su eficacia. El objetivo de este estudio fue evaluar el efecto de tocilizumab en los resultados de los pacientes con neumonía por COVID-19 mediante un análisis de emparejamiento por propensity-score-matching (PSM, «puntuación de propensión»).
Métodos: Se realizó un análisis observacional retrospectivo de los pacientes adultos con COVID-19 ingresados en el Hospital Vall d’Hebron entre marzo y abril de 2020. Se utilizó la regresión logística para analizar el efecto de tocilizumab en la mortalidad, como resultado principal, y el análisis PSM para validar aún más su efecto. Los resultados secundarios fueron la duración de la estancia y la estancia en la unidad de cuidados intensivos (UCI). También se evaluaron los mismos resultados para la administración temprana de tocilizumab, dentro de las 72 h posteriores al ingreso. Los pacientes se seleccionaron mediante el emparejamiento de su propensión individual a recibir tratamiento con tocilizumab, condicionado a sus variables demográficas y clínicas.
Resultados: Se incluyeron 544 pacientes de COVID-19, 197 (36,2%) fueron tratados con tocilizumab, de los cuales 147 fueron tratados dentro de las primeras 72 h tras el ingreso; y 347 fueron incluidos en el grupo control. Tras los análisis PSM, los resultados no mostraron ninguna asociación entre el uso de tocilizumab y la mortalidad global (OR = 1,03; IC del 95%: 0,63-1,68). Sin embargo, se encontró una menor estancia en la UCI en el grupo de tocilizumab en comparación con el grupo de control (coeficiente −4,27; IC del 95%: −6,63 − −1,92). Se encontraron resultados similares en la cohorte de tocilizumab temprano.
Conclusiones: La administración de tocilizumab en pacientes con COVID-19 moderada a grave no redujo el riesgo de mortalidad en nuestra cohorte de pacientes, independientemente del momento de la administración.
Keywords: COVID-19; Mortality; Observational study; Pneumonia; Propensity-score-matched; Tocilizumab.
© 2021 Elsevier España, S.L.U. All rights reserved.
Figures
Comment in
-
Effect of tocilizumab versus standard of care in adults hospitalized with moderate-severe COVID-19 pneumonia.Med Clin (Barc). 2022 Oct 28;159(8):e54. doi: 10.1016/j.medcli.2022.04.018. Epub 2022 Jul 20. Med Clin (Barc). 2022. PMID: 36064504 Free PMC article. English, Spanish. No abstract available.
References
-
- WHO announces COVID-19 outbreak a pandemic. [Internet] Available from: https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus... [accessed 30.12.20].
-
- Quartuccio L., Sonaglia A., Pecori D., Peghin M., Fabris M., Tascini C., et al. Higher levels of IL-6 early after tocilizumab distinguish survivors from nonsurvivors in COVID-19 pneumonia: a possible indication for deeper targeting of IL-6. J Med Virol. 2020;92:2852–2856. doi: 10.1002/jmv.26149. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
