Genotype-independent plant transformation
- PMID: 35531314
- PMCID: PMC9070643
- DOI: 10.1093/hr/uhac047
Genotype-independent plant transformation
Abstract
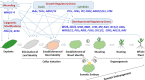
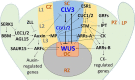
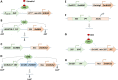
Plant transformation and regeneration remain highly species- and genotype-dependent. Conventional hormone-based plant regeneration via somatic embryogenesis or organogenesis is tedious, time-consuming, and requires specialized skills and experience. Over the last 40 years, significant advances have been made to elucidate the molecular mechanisms underlying embryogenesis and organogenesis. These pioneering studies have led to a better understanding of the key steps and factors involved in plant regeneration, resulting in the identification of crucial growth and developmental regulatory genes that can dramatically improve regeneration efficiency, shorten transformation time, and make transformation of recalcitrant genotypes possible. Co-opting these regulatory genes offers great potential to develop innovative genotype-independent genetic transformation methods for various plant species, including specialty crops. Further developing these approaches has the potential to result in plant transformation without the use of hormones, antibiotics, selectable marker genes, or tissue culture. As an enabling technology, the use of these regulatory genes has great potential to enable the application of advanced breeding technologies such as genetic engineering and gene editing for crop improvement in transformation-recalcitrant crops and cultivars. This review will discuss the recent advances in the use of regulatory genes in plant transformation and regeneration, and their potential to facilitate genotype-independent plant transformation and regeneration.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nanjing Agricultural University.
Figures
Similar articles
-
Promoting genotype-independent plant transformation by manipulating developmental regulatory genes and/or using nanoparticles.Plant Cell Rep. 2023 Sep;42(9):1395-1417. doi: 10.1007/s00299-023-03037-2. Epub 2023 Jun 14. Plant Cell Rep. 2023. PMID: 37311877 Free PMC article. Review.
-
Unlocking regeneration potential: harnessing morphogenic regulators and small peptides for enhanced plant engineering.Plant J. 2025 Jan;121(2):e17193. doi: 10.1111/tpj.17193. Epub 2024 Dec 10. Plant J. 2025. PMID: 39658544 Free PMC article. Review.
-
Dynamic Transcriptome Analysis Reveals Uncharacterized Complex Regulatory Pathway Underlying Genotype-Recalcitrant Somatic Embryogenesis Transdifferentiation in Cotton.Genes (Basel). 2020 May 7;11(5):519. doi: 10.3390/genes11050519. Genes (Basel). 2020. PMID: 32392816 Free PMC article.
-
Application of Developmental Regulators for Enhancing Plant Regeneration and Genetic Transformation.Plants (Basel). 2024 May 4;13(9):1272. doi: 10.3390/plants13091272. Plants (Basel). 2024. PMID: 38732487 Free PMC article. Review.
-
Current progress and challenges in crop genetic transformation.J Plant Physiol. 2021 Jun;261:153411. doi: 10.1016/j.jplph.2021.153411. Epub 2021 Apr 5. J Plant Physiol. 2021. PMID: 33872932 Review.
Cited by
-
Poplar transformation with variable explant sources to maximize transformation efficiency.Sci Rep. 2025 Jan 8;15(1):1320. doi: 10.1038/s41598-024-81235-y. Sci Rep. 2025. PMID: 39779752 Free PMC article.
-
Designing artificial synthetic promoters for accurate, smart, and versatile gene expression in plants.Plant Commun. 2023 Jul 10;4(4):100558. doi: 10.1016/j.xplc.2023.100558. Epub 2023 Feb 9. Plant Commun. 2023. PMID: 36760129 Free PMC article. Review.
-
Tissue culture-independent approaches to revolutionizing plant transformation and gene editing.Hortic Res. 2024 Oct 14;12(2):uhae292. doi: 10.1093/hr/uhae292. eCollection 2025 Jan. Hortic Res. 2024. PMID: 39906168 Free PMC article.
-
Polymeric Nanocarriers Autonomously Cross the Plant Cell Wall and Enable Protein Delivery for Stress Sensing.Adv Mater. 2024 Oct;36(41):e2409356. doi: 10.1002/adma.202409356. Epub 2024 Aug 16. Adv Mater. 2024. PMID: 39149770
-
A Modified Method for Transient Transformation via Pollen Magnetofection in Lilium Germplasm.Int J Mol Sci. 2023 Oct 18;24(20):15304. doi: 10.3390/ijms242015304. Int J Mol Sci. 2023. PMID: 37894985 Free PMC article.
References
-
- Nalapalli S, Tunc-Ozdemir M, Sun Yet al. . Morphogenic regulators and their application in improving plant transformation. In: Bandyopadhyay A, Thilmony R, eds. Rice Genome Engineering and Gene Editing. Methods in Molecular Biology. Vol. 2238. Humana: New York, NY, 2021,37–60. - PubMed
-
- Atta R, Laurens L, Boucheron-Dubuisson Eet al. . Pluripotency of Arabidopsis xylem pericycle underlies shoot regeneration from root and hypocotyl explants grown in vitro. Plant J. 2009;57:626–44. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

