A screening study for COVID-19-associated pulmonary aspergillosis in critically ill patients during the third wave of the pandemic
- PMID: 35531631
- PMCID: PMC9348343
- DOI: 10.1111/myc.13466
A screening study for COVID-19-associated pulmonary aspergillosis in critically ill patients during the third wave of the pandemic
Abstract
Background: COVID-19-associated pulmonary aspergillosis (CAPA) has been reported as an important cause of mortality in critically ill patients with an incidence rate ranging from 5% to 35% during the first and second pandemic waves.
Objectives: We aimed to evaluate the incidence, risk factors for CAPA by a screening protocol and outcome in the critically ill patients during the third wave of the pandemic.
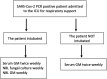
Patients/methods: This prospective cohort study was conducted in two intensive care units (ICU) designated for patients with COVID-19 in a tertiary care university hospital between 18 November 2020 and 24 April 2021. SARS-CoV-2 PCR-positive adult patients admitted to the ICU with respiratory failure were included in the study. Serum and respiratory samples were collected periodically from ICU admission up to CAPA diagnosis, patient discharge or death. ECMM/ISHAM consensus criteria were used to diagnose and classify CAPA cases.
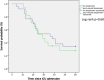
Results: A total of 302 patients were admitted to the two ICUs during the study period, and 213 were included in the study. CAPA was diagnosed in 43 (20.1%) patients (12.2% probable, 7.9% possible). In regression analysis, male sex, higher SOFA scores at ICU admission, invasive mechanical ventilation and longer ICU stay were significantly associated with CAPA development. Overall ICU mortality rate was higher significantly in CAPA group compared to those with no CAPA (67.4% vs 29.4%, p < .001).
Conclusions: One fifth of critically ill patients in COVID-19 ICUs developed CAPA, and this was associated with a high mortality.
Keywords: CAPA; COVID-19; aspergillus; critical care; incidence; intensive care; risk factors; screening.
© 2022 Wiley-VCH GmbH.
Conflict of interest statement
GM gave payment for presentations from Gilead, Pfizer, Merck, Sharp, and Dohme (MSD), 3 M, support for attending meetings from Gilead, Pfizer. SAA gave payment for presentation from Gilead, support for attending meetings Astellas. SBA gave multiple honoraria for lectures unrelated to the manuscript from own university. All other authors have nothing to disclose.
Figures


References
-
- Schauwvlieghe A, Rijnders BJA, Philips N, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6:782‐792. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

