Clinical efficacy and safety of polynucleotides highly purified technology (PN-HPT®) and cross-linked hyaluronic acid for moderate to severe nasolabial folds: A prospective, randomized, exploratory study
- PMID: 35531796
- PMCID: PMC10084116
- DOI: 10.1111/jocd.15064
Clinical efficacy and safety of polynucleotides highly purified technology (PN-HPT®) and cross-linked hyaluronic acid for moderate to severe nasolabial folds: A prospective, randomized, exploratory study
Abstract
Introduction: The mandibular profile undergoes progressive wasting with aging, and the deepening of nasolabial folds (NLFs) has a leading role. Hyaluronic acid (HA) efficiently controls tissue hydration and permeability to small and large molecules. NLFs are an acknowledged HA target; at the same time, another class of agents, PN-HPT® (Polynucleotides Highly Purified Technology), enjoy growing acknowledgement in aesthetic medicine. This exploratory, prospective study probed the rationale of sequentially associating PN-HPT® as a first priming agent acting in the skin followed by HA dermal filler injections for correcting moderate to severe NLFs.
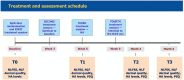
Methods: Following strict inclusion and exclusion criteria, the authors screened Caucasian ambulatory women aged 40-65 with moderate to severe NLFs and randomly selected two NLFs for each enrolled woman. Due to the purely explorative nature of the study, the authors initially planned to enroll no >10 women. According to a split-face design, the selected right-side NLFs received 4 ml of PN-HPT® intradermally in the initial priming phase ("NLF Rx group"); the selected left-side NLFs received 4 ml of saline (placebo) ("NLF Lx group"). After 3 and 6 weeks, all patients received 2 ml of subdermal cross-linked HA over both NLF areas (4 ml overall). The total study follow-up was 6 months after the first injection, with objective assessments, based on the qualitative and quantitative Antera 3D® and Vectra H2® skin imaging technologies, after 6 weeks and 3 and 6 months.
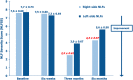
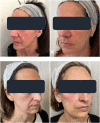
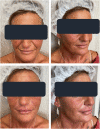
Results: Because of the favorable early outcomes, the authors let enrollment progress between January and June 2020 up to a total of 20 women and 40 NLFs. All treated women completed the six-month follow-up without reporting side effects, even clinically minor. The Antera 3D® device demonstrated that wrinkles and skin texture significantly improved in the NLF Rx after 6 weeks (monotherapy phase) and 3 and 6 months (PN-HPT® priming + HA phase) compared with baseline. HA levels, measured with the quantitative Vectra H2® assessment technology in the right NLFs, were significantly higher than contralaterally at both 3 and 6 months.
Conclusions: Although conceived only as an exploratory investigation, the study confirmed that PN-HPT® monotherapy might be a valuable and effective option to rapidly improve the skin dermis texture and quality in individuals with moderate to severe NLFs. Acting as a priming agent in the skin, PN-HPT® prolong the clinical efficacy of cross-linked HA. Well-designed trials in larger treatment groups will hopefully confirm these early promising results.
Keywords: PN-HPT®; hyaluronic acid; nasolabial folds; polynucleotides highly purified technology.
© 2022 The Authors. Journal of Cosmetic Dermatology published by Wiley Periodicals LLC.
Conflict of interest statement
The manuscript's authors state they have no conflict of interest related to the study, they received no funds, and they have no paid or unpaid relations with industry manufacturers, publishers, or other companies in some way related to their study.
Figures
Similar articles
-
Efficacy and safety of two hyaluronic acid fillers with different injection depths for the correction of moderate-to-severe nasolabial folds: A 52-week, prospective, randomized, double-blinded study in a Chinese population.J Cosmet Dermatol. 2022 Mar;21(3):940-948. doi: 10.1111/jocd.14744. Epub 2022 Jan 12. J Cosmet Dermatol. 2022. PMID: 35020250 Clinical Trial.
-
A 52-week follow-up, multi-center, randomized, double-blinded comparison of efficacy and safety of two hyaluronic acid fillers for the treatment of moderate-to-severe nasolabial folds in Chinese population.J Dermatolog Treat. 2024 Dec;35(1):2378165. doi: 10.1080/09546634.2024.2378165. Epub 2024 Jul 14. J Dermatolog Treat. 2024. PMID: 39004426 Clinical Trial.
-
The efficacy and safety of a monophasic hyaluronic acid filler in the correction of nasolabial folds: A randomized, multicenter, single blinded, split-face study.J Cosmet Dermatol. 2018 Aug;17(4):584-589. doi: 10.1111/jocd.12380. Epub 2017 Sep 14. J Cosmet Dermatol. 2018. PMID: 28913927 Clinical Trial.
-
Monophasic and Biphasic Hyaluronic Acid Fillers for Esthetic Correction of Nasolabial Folds: A Meta-Analysis of Randomized Controlled Trials.Aesthetic Plast Surg. 2022 Jun;46(3):1407-1422. doi: 10.1007/s00266-021-02729-y. Epub 2022 Jan 23. Aesthetic Plast Surg. 2022. PMID: 35066619 Review.
-
Monophasic versus biphasic hyaluronic acid filler for correcting nasolabial folds: a systematic review and meta-analysis.J Cosmet Dermatol. 2022 Feb;21(2):627-635. doi: 10.1111/jocd.14632. Epub 2021 Nov 24. J Cosmet Dermatol. 2022. PMID: 34817919
Cited by
-
The Primacy of Ethics in Aesthetic Medicine: A Review.Plast Reconstr Surg Glob Open. 2024 Jun 25;12(6):e5935. doi: 10.1097/GOX.0000000000005935. eCollection 2024 Jun. Plast Reconstr Surg Glob Open. 2024. PMID: 38919517 Free PMC article.
-
The Effect of Polynucleotide-Hyaluronic Acid Hydrogel in the Recovery After Mechanical Skin Barrier Disruption.Skin Res Technol. 2024 Sep;30(9):e70068. doi: 10.1111/srt.70068. Skin Res Technol. 2024. PMID: 39300806 Free PMC article.
-
Regenerative Aesthetics: A Genuine Frontier or Just a Facet of Regenerative Medicine: A Systematic Review.Aesthetic Plast Surg. 2025 Jan;49(1):341-355. doi: 10.1007/s00266-024-04287-5. Epub 2024 Aug 28. Aesthetic Plast Surg. 2025. PMID: 39198280
-
Polynucleotides High Purification Technology (PN HPTTM) Injection Improves Pain Status and Functional Impairment in Hip and Shoulder Tendinitis.J Clin Med. 2025 Feb 20;14(5):1404. doi: 10.3390/jcm14051404. J Clin Med. 2025. PMID: 40094781 Free PMC article.
-
Manufacturing Process of Hyaluronic Acid Dermal Fillers.Polymers (Basel). 2024 Sep 27;16(19):2739. doi: 10.3390/polym16192739. Polymers (Basel). 2024. PMID: 39408450 Free PMC article. Review.
References
-
- Guida S, Ciardo S, De Pace B, et al. Atrophic and hypertrophic skin photoaging and melanocortin‐1 receptor (MC1R): the missing link. J Am Acad Dermatol. 2021;84:187‐190. - PubMed
-
- Guida S, Ciardo S, De Pace B, et al. The influence of MC1R on dermal morphological features of photo‐exposed skin in women revealed by reflectance confocal microscopy and optical coherence tomography. Exp Dermatol. 2019;28:1321‐1327. - PubMed
-
- Gierloff M, Stohring C, Buder T, et al. Aging changes of the midfacial fat compartments: a computed tomographic study. Plast Reconstr Surg. 2012;129:263‐273. - PubMed
-
- Le Louarn C, Buthiau D, Buis J. Structural aging: the facial recurve concept. Aesthet Plast Surg. 2007;31:213‐218. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

