Oncostatin M promotes lipolysis in white adipocytes
- PMID: 35531859
- PMCID: PMC9116407
- DOI: 10.1080/21623945.2022.2075129
Oncostatin M promotes lipolysis in white adipocytes
Abstract
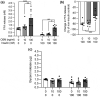
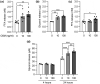
Oncostatin M (OSM) is a member of the glycoprotein 130 cytokine family that is involved in chronic inflammation and increased in adipose tissue under obesity and insulin resistance. OSM was shown to inhibit adipogenesis, suppress browning, and contribute to insulin resistance in cultured white adipocytes. In contrast, OSM may have a metabolically favourable role on adipocytes in mouse models of obesity and insulin resistance. However, a putative role of OSM in modulating lipolysis has not been investigated in detail to date. To address this, cultured white adipocytes of mouse or human origin were exposed to 10 or 100 ng/ml of OSM for various time periods. In murine 3T3-L1 cells, OSM stimulation directly activated hormone-sensitive lipase (HSL) and other players of the lipolytic machinery, and dose-dependently increased free fatty acid and glycerol release. In parallel, OSM attenuated insulin-mediated suppression of lipolysis and induced phosphorylation of serine-residues on the insulin receptor substrate-1 (IRS1) protein. Key experiments were verified in a second murine and a human adipocyte cell line. Inhibiton of extracellular signal-regulated kinase (ERK)-1/2 activation, abolished OSM-mediated HSL phosphorylation and lipolysis. In conclusion, OSM signalling directly promotes lipolysis in white adipocytes in an ERK1/2-dependent manner.
Keywords: Adipocyte; cytokine; glycoprotein 130; insulin resistance; lipolysis; oncostatin M.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures

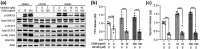
Similar articles
-
Oncostatin M Induces Lipolysis and Suppresses Insulin Response in 3T3-L1 Adipocytes.Int J Mol Sci. 2022 Apr 23;23(9):4689. doi: 10.3390/ijms23094689. Int J Mol Sci. 2022. PMID: 35563078 Free PMC article.
-
Hydroxytyrosol stimulates lipolysis via A-kinase and extracellular signal-regulated kinase activation in 3T3-L1 adipocytes.Eur J Nutr. 2014 Apr;53(3):743-50. doi: 10.1007/s00394-013-0578-7. Epub 2013 Aug 31. Eur J Nutr. 2014. PMID: 23995352
-
Loss of Oncostatin M Signaling in Adipocytes Induces Insulin Resistance and Adipose Tissue Inflammation in Vivo.J Biol Chem. 2016 Aug 12;291(33):17066-76. doi: 10.1074/jbc.M116.739110. Epub 2016 Jun 20. J Biol Chem. 2016. PMID: 27325693 Free PMC article.
-
Adipocyte Oncostatin Receptor Regulates Adipose Tissue Homeostasis and Inflammation.Front Immunol. 2021 Mar 29;11:612013. doi: 10.3389/fimmu.2020.612013. eCollection 2020. Front Immunol. 2021. PMID: 33854494 Free PMC article. Review.
-
Oncostatin M in the development of metabolic syndrome and its potential as a novel therapeutic target.Anat Sci Int. 2018 Mar;93(2):169-176. doi: 10.1007/s12565-017-0421-y. Epub 2017 Nov 4. Anat Sci Int. 2018. PMID: 29103176 Review.
Cited by
-
IL-27 increases energy storage in white adipocytes by enhancing glucose uptake and fatty acid esterification.Adipocyte. 2023 Dec;12(1):2276346. doi: 10.1080/21623945.2023.2276346. Epub 2023 Nov 10. Adipocyte. 2023. PMID: 37948192 Free PMC article.
-
Correlation Between Oncostatin M and Acute Ischemic Stroke: A Case-Control Study.Cureus. 2023 Dec 10;15(12):e50297. doi: 10.7759/cureus.50297. eCollection 2023 Dec. Cureus. 2023. PMID: 38205475 Free PMC article.
-
Excessive or sustained endoplasmic reticulum stress: one of the culprits of adipocyte dysfunction in obesity.Ther Adv Endocrinol Metab. 2024 Oct 7;15:20420188241282707. doi: 10.1177/20420188241282707. eCollection 2024. Ther Adv Endocrinol Metab. 2024. PMID: 39381518 Free PMC article. Review.
References
-
- Hermanns HM. Oncostatin M and interleukin-31: cytokines, receptors, signal transduction and physiology. Cytokine Growth Factor Rev. 2015;26:545–558. - PubMed
-
- Komori T, Morikawa Y. Oncostatin M in the development of metabolic syndrome and its potential as a novel therapeutic target. Anat Sci Int. 2018;93:169–176. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
