Liver vitronectin release into the bloodstream increases due to reduced vagal muscarinic signaling after cerebral stroke in female mice
- PMID: 35531929
- PMCID: PMC9082388
- DOI: 10.14814/phy2.15301
Liver vitronectin release into the bloodstream increases due to reduced vagal muscarinic signaling after cerebral stroke in female mice
Abstract
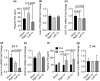
Vitronectin (VTN) is a glycoprotein enriched in the blood and activates integrin receptors. VTN blood levels increase only in female mice 24 h after an ischemic stroke and exacerbate brain injury through IL-6-driven inflammation, but the VTN induction mechanism is unknown. Here, a 30 min middle cerebral artery occlusion (MCAO) in female mice induced VTN protein in the liver (normally the main source) in concert with plasma VTN. Male mice were excluded as VTN is not induced after stroke. MCAO also increased plasma VTN levels after de novo expression of VTN in the liver of VTN-/- female mice, using a hepatocyte-specific (SERPINA1) promoter. MCAO did not affect SERPINA1 or VTN mRNA in the liver, brain, or several peripheral organs, or platelet VTN, compared to sham mice. Thus, hepatocytes are the source of stroke-induced increases in plasma VTN, which is independent of transcription. The cholinergic innervation by the parasympathetic vagus nerve is a potential source of brain-liver signaling after stroke. Right-sided vagotomy at the cervical level led to increased plasma VTN levels, suggesting that VTN release is inhibited by vagal tone. Co-culture of hepatocytes with cholinergic neurons or treatment with acetylcholine, but not noradrenaline (sympathetic transmitter), suppressed VTN expression. Hepatocytes have muscarinic receptors and the M1/M3 agonist bethanechol decreased VTN mRNA and protein release in vitro via M1 receptors. Finally, systemic bethanechol treatment blocked stroke-induced plasma VTN. Thus, VTN translation and release are inhibited by muscarinic signaling from the vagus nerve and presents a novel target for lessening detrimental VTN expression.
Keywords: blood protein; cholinergic; ischemic stroke; mice; vagus nerve; vitronectin.
© 2022 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
None.
Figures


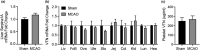


Similar articles
-
Blood Vitronectin Induces Detrimental Brain Interleukin-6 and Correlates With Outcomes After Stroke Only in Female Mice.Stroke. 2020 May;51(5):1587-1595. doi: 10.1161/STROKEAHA.120.029036. Epub 2020 Apr 21. Stroke. 2020. PMID: 32312218 Free PMC article.
-
Vitronectin mitigates stroke-increased neurogenesis only in female mice and through FAK-regulated IL-6.Exp Neurol. 2020 Jan;323:113088. doi: 10.1016/j.expneurol.2019.113088. Epub 2019 Oct 31. Exp Neurol. 2020. PMID: 31678139 Free PMC article.
-
Blood vitronectin is a major activator of LIF and IL-6 in the brain through integrin-FAK and uPAR signaling.J Cell Sci. 2018 Feb 2;131(3):jcs202580. doi: 10.1242/jcs.202580. J Cell Sci. 2018. PMID: 29222114 Free PMC article.
-
Role of Vitronectin and Its Receptors in Neuronal Function and Neurodegenerative Diseases.Int J Mol Sci. 2022 Oct 16;23(20):12387. doi: 10.3390/ijms232012387. Int J Mol Sci. 2022. PMID: 36293243 Free PMC article. Review.
-
Targeting Cholinergic System to Modulate Liver Injury.Curr Drug Targets. 2018;19(8):938-944. doi: 10.2174/1389450118666170619090219. Curr Drug Targets. 2018. PMID: 28625139 Review.
Cited by
-
Vitronectin Destroyed Intestinal Epithelial Cell Differentiation through Activation of PDE4-Mediated Ferroptosis in Inflammatory Bowel Disease.Mediators Inflamm. 2023 Jul 19;2023:6623329. doi: 10.1155/2023/6623329. eCollection 2023. Mediators Inflamm. 2023. PMID: 37501933 Free PMC article.
References
-
- Akmayev, I. G. , Fidelina, O. V. , & Vikhreva, O. V. (1994). The rat dorsal vagal nucleus: Features of cellular and synaptic structure. Acta Biologica Hungarica, 45, 143–153. - PubMed
-
- Alessi, M.‐C. , Nicaud, V. , Scroyen, I. , Lange, C. , Saut, N. , Fumeron, F. , Marre, M. , Lantieri, O. , Fontaine‐Bisson, B. , Juhan‐Vague, I. , Balkau, B. , Tregouet, D.‐A. , & Morange, P.‐E. (2011). Association of vitronectin and plasminogen activator inhibitor‐1 levels with the risk of metabolic syndrome and type 2 diabetes mellitus. Results from the D.E.S.I.R. prospective cohort. Thrombosis and Haemostasis, 106, 416–422. 10.1160/TH11-03-0179 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

