Functional analysis of rare genetic variants in complement factor I in advanced age-related macular degeneration
- PMID: 35531992
- PMCID: PMC9616575
- DOI: 10.1093/hmg/ddac103
Functional analysis of rare genetic variants in complement factor I in advanced age-related macular degeneration
Abstract
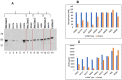
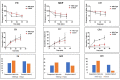
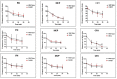
Factor I (FI) is a serine protease inhibitor of the complement system. Heterozygous rare genetic variants in complement factor I (CFI) are associated with advanced age-related macular degeneration (AMD). The clinical impact of these variants is unknown since a majority have not been functionally characterized and are classified as 'variants of uncertain significance' (VUS). This study assessed the functional significance of VUS in CFI. Our previous cross-sectional study using a serum-based assay demonstrated that CFI variants in advanced AMD can be categorized into three types. Type 1 variants cause a quantitative deficiency of FI. Type 2 variants demonstrate a qualitative deficiency. However, Type 3 variants consist of VUS that are less dysfunctional than Types 1 and 2 but are not as biologically active as wild type (WT). In this study, we employed site-directed mutagenesis followed by expression of the recombinant variant and a comprehensive set of functional assays to characterize nine Type 3 variants that were identified in 37 individuals. Our studies establish that the expression of the recombinant protein compared with WT is reduced for R202I, Q217H, S221Y and G263V. Further, G362A and N536K, albeit expressed normally, have significantly less cofactor activity. These results led to re-categorization of CFI variants R202I, Q217H, S221Y and G263V as Type 1 variants and to reclassification of N536K and G362A as Type 2. The variants K441R, Q462H and I492L showed no functional defect and remained as Type 3. This study highlights the utility of an in-depth biochemical analysis in defining the pathologic and clinical implications of complement variants underlying AMD.
© The Author(s) 2022. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures



References
-
- Wong, W.L., Su, X., Li, X., Cheung, C.M., Klein, R., Cheng, C.Y. and Wong, T.Y. (2014) Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob. Health, 2, e106–e116. - PubMed
-
- Seddon, J.M., Yu, Y., Miller, E.C., Reynolds, R., Tan, P.L., Gowrisankar, S., Goldstein, J.I., Triebwasser, M., Anderson, H.E., Zerbib, J. et al. (2013) Rare variants in CFI, C3 and C9 are associated with high risk of advanced age-related macular degeneration. Nat. Genet., 45, 1366–1370. - PMC - PubMed
-
- Kavanagh, D., Yu, Y., Schramm, E.C., Triebwasser, M., Wagner, E.K., Raychaudhuri, S., Daly, M.J., Atkinson, J.P. and Seddon, J.M. (2015) Rare genetic variants in the CFI gene are associated with advanced age-related macular degeneration and commonly result in reduced serum factor I levels. Hum. Mol. Genet., 24, 3861–3870. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

