Hypertension induces gonadal macrophage imbalance, inflammation, lymphangiogenesis, and dysfunction
- PMID: 35532133
- PMCID: PMC9254076
- DOI: 10.1042/CS20220117
Hypertension induces gonadal macrophage imbalance, inflammation, lymphangiogenesis, and dysfunction
Abstract
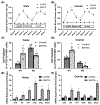
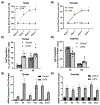
Hypertension (HTN) is associated with gonadal dysfunction and impaired reproductive health in both men and women. An imbalance in the systemic and renal proinflammatory (M1)/anti-inflammatory (M2) macrophage ratio, increased inflammation, and inflammation-associated lymphangiogenesis have been observed in animals with HTN. However, the impact of HTN on gonadal macrophages, inflammation, and lymphatics remains obscure. We hypothesized that salt-sensitive HTN (SSHTN) and HTN alters gonadal macrophage polarization, which is associated with inflammation, inflammation-associated lymphangiogenesis, and reproductive dysfunction. Flow cytometry analyses revealed a significant increase in M1 macrophages in the testes of SSHTN and nitro-L-arginine methyl ester hydrochloride (L-NAME)-induced HTN (LHTN) mice, with a concurrent decrease in M2 macrophages in SSHTN mice yet an increase in M2 macrophages in LHTN mice. Ovaries from SSHTN mice exhibited an increase in M1 and a decrease in M2 macrophages, while ovaries from LHTN mice had a significant increase in M2 and a decrease in M1 macrophages. Gene expression patterns of proinflammatory cytokines revealed gonadal inflammation in all hypertensive mice. Increased lymphatic vessel density in the gonads of both male and female hypertensive mice was confirmed by immunofluorescence staining for lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1). HTN adversely affected the expression pattern of steroidogenic enzymes, hormone receptors, and secretory proteins in both the testes and ovaries. In line with these results, male hypertensive mice also presented with decreased sperm concentration, and increased percentage of sperm with abnormal morphology, damaged acrosome, and nonfunctional mitochondrial activity. These data demonstrate that HTN alters gonadal macrophage polarization, which is associated with gonadal inflammation, inflammation-associated lymphangiogenesis, and dysfunction.
Keywords: Hypertension; Lymphatics; Macrophages; Ovaries; Testes.
© 2022 The Author(s). Published by Portland Press Limited on behalf of the Biochemical Society.
Conflict of interest statement
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Figures




Similar articles
-
Differential changes in end organ immune cells and inflammation in salt-sensitive hypertension: effects of lowering blood pressure.Clin Sci (Lond). 2024 Jul 17;138(14):901-920. doi: 10.1042/CS20240698. Clin Sci (Lond). 2024. PMID: 38949825 Free PMC article.
-
Differential changes in end organ immune cells and inflammation in salt-sensitive hypertension: effects of increasing M2 macrophages.Clin Sci (Lond). 2024 Jul 17;138(14):921-940. doi: 10.1042/CS20240699. Clin Sci (Lond). 2024. PMID: 38949840 Free PMC article.
-
Genetically inducing renal lymphangiogenesis attenuates hypertension in mice.Clin Sci (Lond). 2022 Dec 9;136(23):1759-1772. doi: 10.1042/CS20220547. Clin Sci (Lond). 2022. PMID: 36345993 Free PMC article.
-
Hypertension and reproductive dysfunction: a possible role of inflammation and inflammation-associated lymphangiogenesis in gonads.Clin Sci (Lond). 2020 Dec 23;134(24):3237-3257. doi: 10.1042/CS20201023. Clin Sci (Lond). 2020. PMID: 33346358 Free PMC article. Review.
-
Macrophages: An Inflammatory Link Between Angiogenesis and Lymphangiogenesis.Microcirculation. 2016 Feb;23(2):95-121. doi: 10.1111/micc.12259. Microcirculation. 2016. PMID: 26614117 Free PMC article. Review.
Cited by
-
Hypertension: a lymphatic disease?Clin Sci (Lond). 2025 Jun 23;139(12):597-603. doi: 10.1042/CS20245149. Clin Sci (Lond). 2025. PMID: 40548410 Free PMC article. Review.
-
High-intensity interval training reduces inflammatory mediator levels in the testes of spontaneously hypertensive rats.Anim Reprod. 2025 May 9;22(2):e20240024. doi: 10.1590/1984-3143-AR2024-0024. eCollection 2025. Anim Reprod. 2025. PMID: 40357058 Free PMC article.
-
Differential changes in end organ immune cells and inflammation in salt-sensitive hypertension: effects of lowering blood pressure.Clin Sci (Lond). 2024 Jul 17;138(14):901-920. doi: 10.1042/CS20240698. Clin Sci (Lond). 2024. PMID: 38949825 Free PMC article.
-
Systemic alpha-1 adrenergic receptor inhibition reduces sperm damage in adult and aging spontaneously hypertensive rats.Sci Rep. 2024 Nov 20;14(1):28808. doi: 10.1038/s41598-024-77661-7. Sci Rep. 2024. PMID: 39567544 Free PMC article.
-
Sodium-Directed Crosstalk Between Immune Cells and Lymphatic Vessels.Curr Hypertens Rep. 2025 Jan 15;27(1):7. doi: 10.1007/s11906-024-01322-3. Curr Hypertens Rep. 2025. PMID: 39812718 Free PMC article. Review.
References
-
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M et al. (2015) Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation 131, e29–e322 - PubMed
-
- Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C et al. (2018) 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71, 1269–1324, 10.1161/HYP.0000000000000066 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

