Deploying an In Vitro Gut Model to Assay the Impact of the Mannan-Oligosaccharide Prebiotic Bio-Mos on the Atlantic Salmon (Salmo salar) Gut Microbiome
- PMID: 35532227
- PMCID: PMC9241627
- DOI: 10.1128/spectrum.01953-21
Deploying an In Vitro Gut Model to Assay the Impact of the Mannan-Oligosaccharide Prebiotic Bio-Mos on the Atlantic Salmon (Salmo salar) Gut Microbiome
Abstract
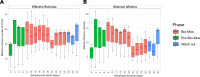
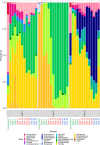
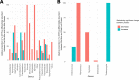
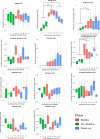
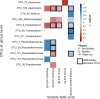
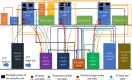
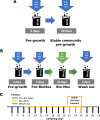
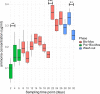
Alpha mannose-oligosaccharide (MOS) prebiotics are widely deployed in animal agriculture as immunomodulators as well as to enhance growth and gut health. Their mode of action is thought to be mediated through their impact on host microbial communities and their associated metabolism. Bio-Mos is a commercially available prebiotic currently used in the agri-feed industry, but studies show contrasting results of its effect on fish performance and feed efficiency. Thus, detailed studies are needed to investigate the effect of MOS supplements on the fish microbiome to enhance our understanding of the link between MOS and gut health. To assess Bio-Mos for potential use as a prebiotic growth promoter in salmonid aquaculture, we have modified an established Atlantic salmon in vitro gut model, SalmoSim, to evaluate its impact on the host microbial communities. The microbial communities obtained from ceca compartments from four adult farmed salmon were inoculated in biological triplicate reactors in SalmoSim. Prebiotic treatment was supplemented for 20 days, followed by a 6-day washout period. Inclusion of Bio-Mos in the media resulted in a significant increase in formate (P = 0.001), propionate (P = 0.037) and 3-methyl butanoic acid (P = 0.024) levels, correlated with increased abundances of several, principally, anaerobic microbial genera (Fusobacterium, Agarivorans, Pseudoalteromonas). DNA metabarcoding with the 16S rDNA marker confirmed a significant shift in microbial community composition in response to Bio-Mos supplementation with observed increase in lactic acid producing Carnobacterium. In conjunction with previous in vivo studies linking enhanced volatile fatty acid production alongside MOS supplementation to host growth and performance, our data suggest that Bio-Mos may be of value in salmonid production. Furthermore, our data highlights the potential role of in vitro gut models to complementin vivo trials of microbiome modulators. IMPORTANCE In this paper we report the results of the impact of a prebiotic (alpha-MOS supplementation) on microbial communities, using an in vitro simulator of the gut microbial environment of the Atlantic salmon. Our data suggest that Bio-Mos may be of value in salmonid production as it enhances volatile fatty acid production by the microbiota from salmon pyloric ceca and correlates with a significant shift in microbial community composition with observed increase in lactic acid producing Carnobacterium. In conjunction with previous in vivo studies linking enhanced volatile fatty acid production alongside MOS supplementation to host growth and performance, our data suggest that Bio-Mos may be of value in salmonid production. Furthermore, our data highlights the potential role of in vitro gut models to augment in vivo trials of microbiome modulators.
Keywords: Atlantic salmon; MOS; NGS; gut model; in vitro; microbiome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- FAO. 2018. The State of Fisheries and Aquaculture in the world 2018Fao.
-
- Adams CE, Turnbull JF, Bell A, Bron JE, Huntingford FA. 2007. Multiple determinants of welfare in farmed fish: stocking density, disturbance, and aggression in Atlantic salmon (Salmo salar). Can J Fish Aquat Sci. doi: 10.1139/F07-018. - DOI
-
- Turnbull J, Bell A, Adams C, Bron J, Huntingford F. 2005. Stocking density and welfare of cage farmed Atlantic salmon: application of a multivariate analysis. Aquaculture 243:121–132. doi: 10.1016/j.aquaculture.2004.09.022. - DOI
-
- Ringø E, Olsen RE, Gifstad T, Dalmo RA, Amlund H, Hemre GI, Bakke AM. 2010. Prebiotics in aquaculture: a review. Aquac Nutr.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

