Induced nanoscale membrane curvature bypasses the essential endocytic function of clathrin
- PMID: 35532382
- PMCID: PMC9093045
- DOI: 10.1083/jcb.202109013
Induced nanoscale membrane curvature bypasses the essential endocytic function of clathrin
Abstract
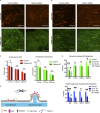
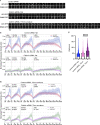
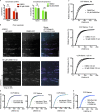
During clathrin-mediated endocytosis (CME), flat plasma membrane is remodeled to produce nanometer-scale vesicles. The mechanisms underlying this remodeling are not completely understood. The ability of clathrin to bind membranes of distinct geometries casts uncertainty on its specific role in curvature generation/stabilization. Here, we used nanopatterning to produce substrates for live-cell imaging, with U-shaped features that bend the ventral plasma membrane of a cell into shapes resembling energetically unfavorable CME intermediates. This induced membrane curvature recruits CME proteins, promoting endocytosis. Upon AP2, FCHo1/2, or clathrin knockdown, CME on flat substrates is severely diminished. However, induced membrane curvature recruits CME proteins in the absence of FCHo1/2 or clathrin and rescues CME dynamics/cargo uptake after clathrin (but not AP2 or FCHo1/2) knockdown. Induced membrane curvature enhances CME protein recruitment upon branched actin assembly inhibition under elevated membrane tension. These data establish that membrane curvature assists in CME nucleation and that the essential function of clathrin during CME is to facilitate curvature evolution, rather than scaffold protein recruitment.
© 2022 Cail et al.
Figures







Comment in
-
Curvature-mediated endocytosis.Nat Rev Mol Cell Biol. 2022 Jul;23(7):447. doi: 10.1038/s41580-022-00497-4. Nat Rev Mol Cell Biol. 2022. PMID: 35577988 No abstract available.
-
Clathrin: Bender or bystander?J Cell Biol. 2022 Jul 4;221(7):e202206014. doi: 10.1083/jcb.202206014. Epub 2022 Jun 15. J Cell Biol. 2022. PMID: 35704021 Free PMC article.
Similar articles
-
Regulation of Clathrin-Mediated Endocytosis.Annu Rev Biochem. 2018 Jun 20;87:871-896. doi: 10.1146/annurev-biochem-062917-012644. Epub 2018 Apr 16. Annu Rev Biochem. 2018. PMID: 29661000 Free PMC article. Review.
-
The AP2 adaptor enhances clathrin coat stiffness.FEBS J. 2019 Oct;286(20):4074-4085. doi: 10.1111/febs.14961. Epub 2019 Jul 3. FEBS J. 2019. PMID: 31199077 Free PMC article.
-
Regulation of clathrin-mediated endocytosis by hierarchical allosteric activation of AP2.J Cell Biol. 2017 Jan 2;216(1):167-179. doi: 10.1083/jcb.201608071. Epub 2016 Dec 21. J Cell Biol. 2017. PMID: 28003333 Free PMC article.
-
Hotspots organize clathrin-mediated endocytosis by efficient recruitment and retention of nucleating resources.Traffic. 2011 Dec;12(12):1868-78. doi: 10.1111/j.1600-0854.2011.01273.x. Epub 2011 Sep 30. Traffic. 2011. PMID: 21883765 Free PMC article.
-
Evolving models for assembling and shaping clathrin-coated pits.J Cell Biol. 2020 Sep 7;219(9):e202005126. doi: 10.1083/jcb.202005126. J Cell Biol. 2020. PMID: 32770195 Free PMC article. Review.
Cited by
-
Cryo-electron tomography pipeline for plasma membranes.Nat Commun. 2025 Jan 20;16(1):855. doi: 10.1038/s41467-025-56045-z. Nat Commun. 2025. PMID: 39833141 Free PMC article.
-
Endocytic myosin-1 is a force-insensitive, power-generating motor.bioRxiv [Preprint]. 2023 Jun 30:2023.03.21.533689. doi: 10.1101/2023.03.21.533689. bioRxiv. 2023. Update in: J Cell Biol. 2023 Oct 2;222(10):e202303095. doi: 10.1083/jcb.202303095. PMID: 36993306 Free PMC article. Updated. Preprint.
-
Imaging nanoscale axial dynamics at the basal plasma membrane.Int J Biochem Cell Biol. 2023 Mar;156:106349. doi: 10.1016/j.biocel.2022.106349. Epub 2022 Dec 22. Int J Biochem Cell Biol. 2023. PMID: 36566777 Free PMC article. Review.
-
Saddle curvature association of nsP1 facilitates the replication complex assembly of Chikungunya virus in cells.Nat Commun. 2025 May 8;16(1):4282. doi: 10.1038/s41467-025-59402-0. Nat Commun. 2025. PMID: 40341088 Free PMC article.
-
Dynamin1 long- and short-tail isoforms exploit distinct recruitment and spatial patterns to form endocytic nanoclusters.Nat Commun. 2024 May 14;15(1):4060. doi: 10.1038/s41467-024-47677-8. Nat Commun. 2024. PMID: 38744819 Free PMC article.
References
-
- Bender, M., Otto M., Hadam B., Spangenberg B., and Kurz H.. 2002. Multiple imprinting in UV-based nanoimprint lithography: Related material issues. Microelectron. Eng. 61–62:407–413. 10.1016/S0167-9317(02)00470-7 - DOI

