Safety, Tolerability, and Immunogenicity of the ACI-24 Vaccine in Adults With Down Syndrome: A Phase 1b Randomized Clinical Trial
- PMID: 35532913
- PMCID: PMC9086937
- DOI: 10.1001/jamaneurol.2022.0983
Safety, Tolerability, and Immunogenicity of the ACI-24 Vaccine in Adults With Down Syndrome: A Phase 1b Randomized Clinical Trial
Abstract
Importance: Individuals with Down syndrome (DS) are at high risk of developing Alzheimer disease due to an increased dose of the amyloid precursor protein gene, APP, which leads to increased levels of full-length APP and its products, including amyloid-β (Aβ). The liposome-based antiamyloid ACI-24 vaccine is intended to treat neurological disorders caused by misfolded Aβ pathological protein. However, the safety, tolerability, and immunogenicity of the ACI-24 vaccine among adults with DS have not been fully examined.
Objective: To assess the safety and tolerability of the ACI-24 vaccine among adults with DS as well as its ability to induce immunogenicity measured by anti-Aβ immunoglobulin G titers.
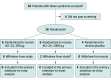
Design, setting, and participants: This multicenter double-blind placebo-controlled dose-escalation phase 1b randomized clinical trial was conducted at 3 US academic medical centers with affiliated Down syndrome clinics between March 30, 2016, and June 29, 2020. A total of 20 adults with DS were screened; of those, 16 adults were eligible to participate. Eligibility criteria included men or women aged 25 to 45 years with cytogenetic diagnosis of either trisomy 21 or complete unbalanced translocation of chromosome 21. Between April 27, 2016, and July 2, 2018, participants were randomized 3:1 into 2 dose-level cohorts (8 participants per cohort, with 6 participants receiving the ACI-24 vaccine and 2 receiving placebo) in a 96-week study. Participants received 48 weeks of treatment followed by an additional 48 weeks of safety follow-up.
Interventions: Participants were randomized to receive 7 subcutaneous injections of ACI-24, 300 μg or 1000 μg, or placebo.
Main outcomes and measures: Primary outcomes were measures of safety and tolerability as well as antibody titers.
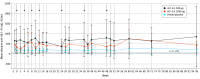
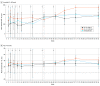
Results: Among 16 enrolled participants, the mean (SD) age was 32.6 (4.4) years; 9 participants were women, and 7 were men. All participants were White, and 1 participant had Hispanic or Latino ethnicity. Treatment adherence was 100%. There were no cases of meningoencephalitis, death, or other serious adverse events (AEs) and no withdrawals as a result of AEs. Most treatment-emergent AEs were of mild intensity (110 of 132 events [83.3%]) and unrelated or unlikely to be related to the ACI-24 vaccine (113 of 132 events [85.6%]). No amyloid-related imaging abnormalities with edema or cerebral microhemorrhage and no evidence of central nervous system inflammation were observed on magnetic resonance imaging scans. Increases in anti-Aβ immunoglobulin G titers were observed in 4 of 12 participants (33.3%) receiving ACI-24 (2 receiving 300 μg and 2 receiving 1000 μg) compared with 0 participants receiving placebo. In addition, a greater increase was observed in plasma Aβ1-40 and Aβ1-42 levels among individuals receiving ACI-24.
Conclusions and relevance: In this study, the ACI-24 vaccine was safe and well tolerated in adults with DS. Evidence of immunogenicity along with pharmacodynamic and target engagement were observed, and anti-Aβ antibody titers were not associated with any adverse findings. These results support progression to clinical trials using an optimized formulation of the ACI-24 vaccine among individuals with DS.
Trial registration: ClinicalTrials.gov Identifier: NCT02738450.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

