Time to Clinical Benefit of Intensive Blood Pressure Lowering in Patients 60 Years and Older With Hypertension: A Secondary Analysis of Randomized Clinical Trials
- PMID: 35532917
- PMCID: PMC9086939
- DOI: 10.1001/jamainternmed.2022.1657
Time to Clinical Benefit of Intensive Blood Pressure Lowering in Patients 60 Years and Older With Hypertension: A Secondary Analysis of Randomized Clinical Trials
Erratum in
-
Error in Key Points.JAMA Intern Med. 2024 May 1;184(5):589. doi: 10.1001/jamainternmed.2022.2852. JAMA Intern Med. 2024. PMID: 35788824 Free PMC article. No abstract available.
Abstract
Importance: Recent guidelines recommend a systolic blood pressure (BP) goal of less than 150 mm Hg or even 130 mm Hg for adults aged 60 years or older. However, harms from intensive BP treatments occur immediately (eg, syncope, fall), and benefits for cardiovascular event reduction emerge over time. Therefore, harms with low chance of benefit need to be clearer, particularly for those with limited life expectancy.
Objective: To estimate the time needed to potentially derive clinical benefit from intensive BP treatment in patients 60 years and older.
Design, setting, and participants: This secondary analysis included individual patient data from published randomized clinical trials with 27 414 patients 60 years or older with hypertension. Patient-level survival data were reconstructed when the original data were not available. Published trials were identified by searching PubMed until October 15, 2021.
Exposures: Intensive BP lowering vs standard BP lowering with the treat-to-target design.
Main outcomes and measures: Major adverse cardiovascular event (MACE) defined by each trial, which was broadly similar with all trials including myocardial infarction, stroke, and cardiovascular mortality.
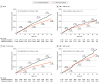
Results: Six trials (original data from 2 trials and reconstructed data from 4 trials) with 27 414 participants (mean age, 70 years; 56.3% were women) were included in the analysis. Intensive BP treatment with a systolic BP target below 140 mm Hg was significantly associated with a 21% reduction in MACE (hazard ratio, 0.79; 95% CI, 0.71-0.88; P < .001). On average, 9.1 (95% CI, 4.0-20.6) months were needed to prevent 1 MACE per 500 patients with the intensive BP treatment (absolute risk reduction [ARR], 0.002). Likewise, 19.1 (95% CI, 10.9-34.2) and 34.4 (95% CI, 22.7-59.8) months were estimated to avoid 1 MACE per 200 (ARR, 0.005) and 100 (ARR, 0.01) patients, respectively.
Conclusions and relevance: In this analysis, findings suggest that for patients 60 years and older with hypertension, intensive BP treatment may be appropriate for some adults with a life expectancy of greater than 3 years but may not be suitable for those with less than 1 year.
Conflict of interest statement
Figures
References
-
- Blood Pressure Lowering Treatment Trialists’ Collaboration . Age-stratified and blood-pressure-stratified effects of blood-pressure-lowering pharmacotherapy for the prevention of cardiovascular disease and death: an individual participant-level data meta-analysis. Lancet. 2021;398(10305):1053-1064. doi: 10.1016/S0140-6736(21)01921-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

