Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome Associated with COVID-19: An Emulated Target Trial Analysis
- PMID: 35533052
- PMCID: PMC9890253
- DOI: 10.1164/rccm.202111-2495OC
Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome Associated with COVID-19: An Emulated Target Trial Analysis
Abstract
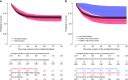
Rationale: Whether patients with coronavirus disease (COVID-19) may benefit from extracorporeal membrane oxygenation (ECMO) compared with conventional invasive mechanical ventilation (IMV) remains unknown. Objectives: To estimate the effect of ECMO on 90-day mortality versus IMV only. Methods: Among 4,244 critically ill adult patients with COVID-19 included in a multicenter cohort study, we emulated a target trial comparing the treatment strategies of initiating ECMO versus no ECMO within 7 days of IMV in patients with severe acute respiratory distress syndrome (PaO2/FiO2 < 80 or PaCO2 ⩾ 60 mm Hg). We controlled for confounding using a multivariable Cox model on the basis of predefined variables. Measurements and Main Results: A total of 1,235 patients met the full eligibility criteria for the emulated trial, among whom 164 patients initiated ECMO. The ECMO strategy had a higher survival probability on Day 7 from the onset of eligibility criteria (87% vs. 83%; risk difference, 4%; 95% confidence interval, 0-9%), which decreased during follow-up (survival on Day 90: 63% vs. 65%; risk difference, -2%; 95% confidence interval, -10 to 5%). However, ECMO was associated with higher survival when performed in high-volume ECMO centers or in regions where a specific ECMO network organization was set up to handle high demand and when initiated within the first 4 days of IMV and in patients who are profoundly hypoxemic. Conclusions: In an emulated trial on the basis of a nationwide COVID-19 cohort, we found differential survival over time of an ECMO compared with a no-ECMO strategy. However, ECMO was consistently associated with better outcomes when performed in high-volume centers and regions with ECMO capacities specifically organized to handle high demand.
Keywords: COVID-19; SARS-CoV-2; acute respiratory distress syndrome; emulated target trial; extracorporeal membrane oxygenation.
Figures

Comment in
-
Turning the Page on Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome due to Severe COVID-19.Am J Respir Crit Care Med. 2022 Aug 1;206(3):236-239. doi: 10.1164/rccm.202205-0906ED. Am J Respir Crit Care Med. 2022. PMID: 35608543 Free PMC article. No abstract available.
References
-
- Barbaro RP, MacLaren G, Boonstra PS, Iwashyna TJ, Slutsky AS, Fan E, et al. Extracorporeal Life Support Organization Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet . 2020;396:1071–1078. - PMC - PubMed
-
- Schmidt M, Hajage D, Lebreton G, Monsel A, Voiriot G, Levy D, et al. Groupe de Recherche Clinique en REanimation et Soins intensifs du Patient en Insuffisance Respiratoire aiguE (GRC-RESPIRE) Sorbonne Université Paris-Sorbonne ECMO-COVID investigators. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study. Lancet Respir Med . 2020;8:1121–1131. - PMC - PubMed
-
- Lebreton G, Schmidt M, Ponnaiah M, Folliguet T, Para M, Guihaire J, et al. Paris ECMO-COVID-19 Investigators Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID-19 pandemic in Greater Paris, France: a multicentre cohort study. Lancet Respir Med . 2021;9:851–862. - PMC - PubMed
-
- Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al. EOLIA Trial Group, REVA, and ECMONet Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med . 2018;378:1965–1975. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

