The Progress and Promise of RNA Medicine─An Arsenal of Targeted Treatments
- PMID: 35533054
- PMCID: PMC9115888
- DOI: 10.1021/acs.jmedchem.2c00024
The Progress and Promise of RNA Medicine─An Arsenal of Targeted Treatments
Abstract
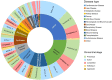
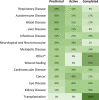
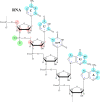
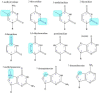
In the past decade, there has been a shift in research, clinical development, and commercial activity to exploit the many physiological roles of RNA for use in medicine. With the rapid success in the development of lipid-RNA nanoparticles for mRNA vaccines against COVID-19 and with several approved RNA-based drugs, RNA has catapulted to the forefront of drug research. With diverse functions beyond the role of mRNA in producing antigens or therapeutic proteins, many classes of RNA serve regulatory roles in cells and tissues. These RNAs have potential as new therapeutics, with RNA itself serving as either a drug or a target. Here, based on the CAS Content Collection, we provide a landscape view of the current state and outline trends in RNA research in medicine across time, geography, therapeutic pipelines, chemical modifications, and delivery mechanisms.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Messenger RNA-Based Therapeutics and Vaccines: What's beyond COVID-19?ACS Pharmacol Transl Sci. 2023 Jul 3;6(7):943-969. doi: 10.1021/acsptsci.3c00047. eCollection 2023 Jul 14. ACS Pharmacol Transl Sci. 2023. PMID: 37470024 Free PMC article. Review.
-
Current Status and Future Perspectives on MRNA Drug Manufacturing.Mol Pharm. 2022 Apr 4;19(4):1047-1058. doi: 10.1021/acs.molpharmaceut.2c00010. Epub 2022 Mar 3. Mol Pharm. 2022. PMID: 35238565 Review.
-
Developing Biodegradable Lipid Nanoparticles for Intracellular mRNA Delivery and Genome Editing.Acc Chem Res. 2021 Nov 2;54(21):4001-4011. doi: 10.1021/acs.accounts.1c00500. Epub 2021 Oct 20. Acc Chem Res. 2021. PMID: 34668716 Review.
-
Synthetic modified messenger RNA for therapeutic applications.Acta Biomater. 2021 Sep 1;131:1-15. doi: 10.1016/j.actbio.2021.06.020. Epub 2021 Jun 13. Acta Biomater. 2021. PMID: 34133982 Free PMC article. Review.
-
The Rapid Development and Early Success of Covid 19 Vaccines Have Raised Hopes for Accelerating the Cancer Treatment Mechanism.Arch Razi Inst. 2021 Mar;76(1):1-6. doi: 10.22092/ari.2021.353761.1612. Epub 2021 Mar 1. Arch Razi Inst. 2021. PMID: 33818952 Free PMC article.
Cited by
-
EIF4A3-mediated biogenesis of circSTX6 promotes bladder cancer metastasis and cisplatin resistance.J Exp Clin Cancer Res. 2024 Jan 2;43(1):2. doi: 10.1186/s13046-023-02932-6. J Exp Clin Cancer Res. 2024. PMID: 38163881 Free PMC article.
-
Biomaterial-based gene therapy.MedComm (2020). 2023 Jun 3;4(3):e259. doi: 10.1002/mco2.259. eCollection 2023 Jun. MedComm (2020). 2023. PMID: 37284583 Free PMC article. Review.
-
Structural heterogeneity and dynamics in the apical stem loop of s2m from SARS-CoV-2 Delta by an integrative NMR spectroscopy and MD simulation approach.Nucleic Acids Res. 2025 Jun 20;53(12):gkaf552. doi: 10.1093/nar/gkaf552. Nucleic Acids Res. 2025. PMID: 40586311 Free PMC article.
-
RNA modifications in cellular metabolism: implications for metabolism-targeted therapy and immunotherapy.Signal Transduct Target Ther. 2024 Mar 27;9(1):70. doi: 10.1038/s41392-024-01777-5. Signal Transduct Target Ther. 2024. PMID: 38531882 Free PMC article. Review.
-
Breaking the mold with RNA-a "RNAissance" of life science.NPJ Genom Med. 2024 Jan 9;9(1):2. doi: 10.1038/s41525-023-00387-4. NPJ Genom Med. 2024. PMID: 38195675 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

