The Overlooked Photochemistry of Iodine in Aqueous Suspensions of Fullerene Derivatives
- PMID: 35533084
- PMCID: PMC9134498
- DOI: 10.1021/acsnano.2c02281
The Overlooked Photochemistry of Iodine in Aqueous Suspensions of Fullerene Derivatives
Abstract
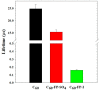
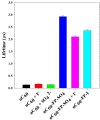
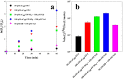
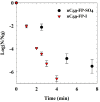
Fullerene's low water solubility was a serious challenge to researchers aiming to harness their excellent photochemical properties for aqueous applications. Cationic functionalization of the fullerene cage provided the most effective approach to increase water solubility, but common synthesis practices inadvertently complicated the photochemistry of these systems by introducing iodide as a counterion. This problem was overlooked until recent work noted a potentiation effect which occurred when photosensitizers were used to inactivate microorganisms with added potassium iodide. In this work, several photochemical pathways were explored to determine the extent and underlying mechanisms of iodide's interference in the photosensitization of singlet oxygen by cationic fulleropyrrolidinium ions and rose bengal. Triplet excited state sensitizer lifetimes were measured via laser flash photolysis to probe the role of I- in triplet sensitizer quenching. Singlet oxygen production rates were compared across sensitizers in the presence or absence of I-, SO42-, and other anions. 3,5-Dimethyl-1H-pyrazole was employed as a chemical probe for iodine radical species, such as I·, but none were observed in the photochemical systems. Molecular iodine and triiodide, however, were found in significant quantities when photosensitizers were irradiated in the presence of I- and O2. The formation of I2 in these photochemical systems calls into question the interpretations of prior studies that used I- as a counterion for photosensitizer materials. As an example, MS2 bacteriophages were inactivated here by cationic fullerenes with and without I- present, showing that I- moderately accelerated the MS2 deactivation, likely by producing I2. Production of I2 did not appear to be directly correlated with estimates of 1O2 concentration, suggesting that the relevant photochemical pathways are more complex than direct reactions between 1O2 and I- in the bulk solution. On the basis of the results here, iodine photochemistry may be underappreciated and misunderstood in other environmental systems.
Keywords: C60; Iodine; MS2 bacteriophage; cationic fullerene; photochemistry; photosensitizer; singlet oxygen.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Hamano T.; Okuda K.; Mashino T.; Hirobe M.; Arakane K.; Ryu A.; Mashiko S.; Nagano T. Singlet oxygen production from fullerene derivatives: Effect of sequential functionalization of the fullerene core. Chem. Commun. 1997, 1, 21–22. 10.1039/a606335g. - DOI
-
- Bosi S.; Da Ros T.; Spalluto G.; Prato M. Fullerene derivatives: an attractive tool for biological applications. European journal of medicinal chemistry 2003, 38 (11–12), 913–923. 10.1016/j.ejmech.2003.09.005. - DOI - PubMed
-
Research Support, Non-U.S. Gov’t Review.
- Fujitsuka M.; Watanabe A.; Ito O.; Yamamoto K.; Funasaka H. Laser flash photolysis study on photochemical generation of radical cations of fullerenes C-60, C-70, and C-76. J. Phys. Chem. A 1997, 101 (43), 7960–7964. 10.1021/jp971850c. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

