Multi-omics analysis of the cervical epithelial integrity of women using depot medroxyprogesterone acetate
- PMID: 35533147
- PMCID: PMC9119532
- DOI: 10.1371/journal.ppat.1010494
Multi-omics analysis of the cervical epithelial integrity of women using depot medroxyprogesterone acetate
Abstract
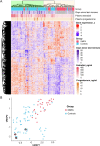
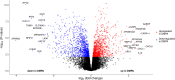
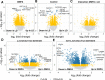
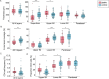
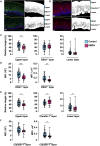
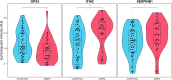
Depot medroxyprogesterone acetate (DMPA) is an injectable hormonal contraceptive used by millions of women worldwide. However, experimental studies have associated DMPA use with genital epithelial barrier disruption and mucosal influx of human immunodeficiency virus (HIV) target cells. We explored the underlying molecular mechanisms of these findings. Ectocervical biopsies and cervicovaginal lavage (CVL) specimens were collected from HIV-seronegative Kenyan sex workers using DMPA (n = 32) or regularly cycling controls (n = 64). Tissue samples were assessed by RNA-sequencing and quantitative imaging analysis, whereas protein levels were measured in CVL samples. The results suggested a DMPA-associated upregulation of genes involved in immune regulation, including genes associated with cytokine-mediated signaling and neutrophil-mediated immunity. A transcription factor analysis further revealed DMPA-associated upregulation of RELA and NFKB1 which are involved in several immune activation pathways. Several genes significantly downregulated in the DMPA versus the control group were involved in epithelial structure and function, including genes encoding keratins, small proline-rich proteins, and cell-cell adhesion proteins. Pathway analyses indicated DMPA use was associated with immune activation and suppression of epithelium development, including keratinization and cornification processes. The cervicovaginal microbiome composition (Lactobacillus dominant and non-Lactobacillus dominant) had no overall interactional impact on the DMPA associated tissue gene expression. Imaging analysis verified that DMPA use was associated with an impaired epithelial layer as illustrated by staining for the selected epithelial junction proteins E-cadherin, desmoglein-1 and claudin-1. Additional staining for CD4+ cells revealed a more superficial location of these cells in the ectocervical epithelium of DMPA users versus controls. Altered protein levels of SERPINB1 and ITIH2 were further observed in the DMPA group. Identification of specific impaired epithelial barrier structures at the gene expression level, which were verified at the functional level by tissue imaging analysis, illustrates mechanisms by which DMPA adversely may affect the integrity of the genital mucosa.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- UNAIDS. Fact sheet—Latest global and regional statistics on the status of the AIDS epidemic 2021 [cited 15 June 2021]. Available from: https://www.unaids.org/en/resources/documents/2021/UNAIDS_FactSheet.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

