GraFT: Graph Filtered Temporal Dictionary Learning for Functional Neural Imaging
- PMID: 35533160
- PMCID: PMC9278524
- DOI: 10.1109/TIP.2022.3171414
GraFT: Graph Filtered Temporal Dictionary Learning for Functional Neural Imaging
Abstract
Optical imaging of calcium signals in the brain has enabled researchers to observe the activity of hundreds-to-thousands of individual neurons simultaneously. Current methods predominantly use morphological information, typically focusing on expected shapes of cell bodies, to better identify neurons in the field-of-view. The explicit shape constraints limit the applicability of automated cell identification to other important imaging scales with more complex morphologies, e.g., dendritic or widefield imaging. Specifically, fluorescing components may be broken up, incompletely found, or merged in ways that do not accurately describe the underlying neural activity. Here we present Graph Filtered Temporal Dictionary (GraFT), a new approach that frames the problem of isolating independent fluorescing components as a dictionary learning problem. Specifically, we focus on the time-traces-the main quantity used in scientific discovery-and learn a time trace dictionary with the spatial maps acting as the presence coefficients encoding which pixels the time-traces are active in. Furthermore, we present a novel graph filtering model which redefines connectivity between pixels in terms of their shared temporal activity, rather than spatial proximity. This model greatly eases the ability of our method to handle data with complex non-local spatial structure. We demonstrate important properties of our method, such as robustness to morphology, simultaneously detecting different neuronal types, and implicitly inferring number of neurons, on both synthetic data and real data examples. Specifically, we demonstrate applications of our method to calcium imaging both at the dendritic, somatic, and widefield scales.
Figures
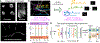





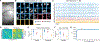
Similar articles
-
Neuronal Graphs: A Graph Theory Primer for Microscopic, Functional Networks of Neurons Recorded by Calcium Imaging.Front Neural Circuits. 2021 Jun 10;15:662882. doi: 10.3389/fncir.2021.662882. eCollection 2021. Front Neural Circuits. 2021. PMID: 34177469 Free PMC article. Review.
-
Discriminative dictionary learning algorithm with pairwise local constraints for histopathological image classification.Med Biol Eng Comput. 2021 Jan;59(1):153-164. doi: 10.1007/s11517-020-02281-y. Epub 2021 Jan 2. Med Biol Eng Comput. 2021. PMID: 33386592
-
Interpretable online network dictionary learning for inferring long-range chromatin interactions.PLoS Comput Biol. 2024 May 16;20(5):e1012095. doi: 10.1371/journal.pcbi.1012095. eCollection 2024 May. PLoS Comput Biol. 2024. PMID: 38753877 Free PMC article.
-
Sparse SPM: Group Sparse-dictionary learning in SPM framework for resting-state functional connectivity MRI analysis.Neuroimage. 2016 Jan 15;125:1032-1045. doi: 10.1016/j.neuroimage.2015.10.081. Epub 2015 Oct 31. Neuroimage. 2016. PMID: 26524138
-
Optical Probes for Neurobiological Sensing and Imaging.Acc Chem Res. 2018 May 15;51(5):1023-1032. doi: 10.1021/acs.accounts.7b00564. Epub 2018 Apr 13. Acc Chem Res. 2018. PMID: 29652127 Free PMC article. Review.
Cited by
-
Dendritic excitations govern back-propagation via a spike-rate accelerometer.Nat Commun. 2025 Feb 4;16(1):1333. doi: 10.1038/s41467-025-55819-9. Nat Commun. 2025. PMID: 39905023 Free PMC article.
-
Dendritic excitations govern back-propagation via a spike-rate accelerometer.bioRxiv [Preprint]. 2024 May 18:2023.06.02.543490. doi: 10.1101/2023.06.02.543490. bioRxiv. 2024. Update in: Nat Commun. 2025 Feb 04;16(1):1333. doi: 10.1038/s41467-025-55819-9. PMID: 37398232 Free PMC article. Updated. Preprint.
-
Long-wavelength traveling waves of vasomotion modulate the perfusion of cortex.Neuron. 2024 Jul 17;112(14):2349-2367.e8. doi: 10.1016/j.neuron.2024.04.034. Epub 2024 May 22. Neuron. 2024. PMID: 38781972 Free PMC article.
-
Chronic brain functional ultrasound imaging in freely moving rodents performing cognitive tasks.J Neurosci Methods. 2024 Mar;403:110033. doi: 10.1016/j.jneumeth.2023.110033. Epub 2023 Dec 4. J Neurosci Methods. 2024. PMID: 38056633 Free PMC article.
-
Fast Two-photon Microscopy by Neuroimaging with Oblong Random Acquisition (NORA).ArXiv [Preprint]. 2025 Jun 9:arXiv:2503.15487v2. ArXiv. 2025. PMID: 40166740 Free PMC article. Preprint.
References
-
- Denk W, Strickler JH, and Webb WW, “Two-photon laser scanning fluorescence microscopy,” Science, vol. 248, no. 4951, pp. 73–6, 1990. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/2321027 - PubMed
-
- Mank M. and Griesbeck O, “Genetically encoded calcium indicators,” Chem Rev, vol. 108, no. 5, pp. 1550–64, 2008. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/18447377 - PubMed
-
- Tian L, Akerboom J, Schreiter ER, and Looger LL, “Neural activity imaging with genetically encoded calcium indicators,” Prog Brain Res, vol. 196, pp. 79–94, 2012. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/22341322 - PubMed

