MUC16 Promotes Liver Metastasis of Pancreatic Ductal Adenocarcinoma by Upregulating NRP2-Associated Cell Adhesion
- PMID: 35533267
- PMCID: PMC9635595
- DOI: 10.1158/1541-7786.MCR-21-0888
MUC16 Promotes Liver Metastasis of Pancreatic Ductal Adenocarcinoma by Upregulating NRP2-Associated Cell Adhesion
Abstract
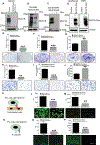
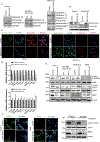
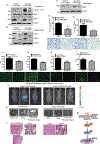
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal types of cancer, as it commonly metastasizes to the liver resulting in an overall poor prognosis. However, the molecular mechanism involved in liver metastasis remains poorly understood. Here, we aimed to identify the MUC16-mediated molecular mechanism of PDAC-liver metastasis. Previous studies demonstrated that MUC16 and its C-terminal (Cter) domain are involved in the aggressiveness of PDAC. In this study, we observed MUC16 and its Cter expression significantly high in human PDAC tissues, PDAC organoids, and metastatic liver tissues, while no expression was observed in normal pancreatic tissues using IHC and immunofluorescence (IFC) analyses. MUC16 knockdown in SW1990 and CD18/HPAF PDAC cells significantly decreased the colony formation, migration, and endothelial/p-selectin binding. In contrast, MUC16-Cter ectopic overexpression showed significantly increased colony formation and motility in MiaPaCa2 pancreatic cancer cells. Interestingly, MUC16 promoted cell survival and colonization in the liver, mimicking an ex vivo environment. Furthermore, MUC16 enhanced liver metastasis in the in vivo mouse model. Our integrated analyses of RNA-sequencing suggested that MUC16 alters Neuropilin-2 (NRP2) and cell adhesion molecules in pancreatic cancer cells. Furthermore, we identified that MUC16 regulated NRP2 via JAK2/STAT1 signaling in PDAC. NRP2 knockdown in MUC16-overexpressed PDAC cells showed significantly decreased cell adhesion and migration. Overall, the findings indicate that MUC16 regulates NRP2 and induces metastasis in PDAC.
Implications: This study shows that MUC16 plays a critical role in PDAC liver metastasis by mediating NRP2 regulation by JAK2/STAT1 axis, thereby paving the way for future therapy efforts for metastatic PDAC.
©2022 American Association for Cancer Research.
Conflict of interest statement
Figures



Similar articles
-
Carboxyl-terminal domain of MUC16 imparts tumorigenic and metastatic functions through nuclear translocation of JAK2 to pancreatic cancer cells.Oncotarget. 2015 Mar 20;6(8):5772-87. doi: 10.18632/oncotarget.3308. Oncotarget. 2015. PMID: 25691062 Free PMC article.
-
MUC16 contributes to the metastasis of pancreatic ductal adenocarcinoma through focal adhesion mediated signaling mechanism.Genes Cancer. 2016 Mar;7(3-4):110-124. doi: 10.18632/genesandcancer.104. Genes Cancer. 2016. PMID: 27382435 Free PMC article.
-
Isoforms of MUC16 activate oncogenic signaling through EGF receptors to enhance the progression of pancreatic cancer.Mol Ther. 2021 Apr 7;29(4):1557-1571. doi: 10.1016/j.ymthe.2020.12.029. Epub 2020 Dec 25. Mol Ther. 2021. PMID: 33359791 Free PMC article.
-
Organoids, tissue slices and organotypic cultures: Advancing our understanding of pancreatic ductal adenocarcinoma through in vitro and ex vivo models.Semin Cancer Biol. 2025 Feb;109:10-24. doi: 10.1016/j.semcancer.2024.12.003. Epub 2024 Dec 25. Semin Cancer Biol. 2025. PMID: 39730107 Review.
-
Isolated pulmonary metastases in pancreatic ductal adenocarcinoma: a review of current evidence.Cancer Biol Ther. 2023 Dec 31;24(1):2198479. doi: 10.1080/15384047.2023.2198479. Cancer Biol Ther. 2023. PMID: 37526431 Free PMC article. Review.
Cited by
-
Role of Neuropilin-2-mediated signaling axis in cancer progression and therapy resistance.Cancer Metastasis Rev. 2022 Sep;41(3):771-787. doi: 10.1007/s10555-022-10048-0. Epub 2022 Jul 1. Cancer Metastasis Rev. 2022. PMID: 35776228 Free PMC article. Review.
-
MUC16: clinical targets with great potential.Clin Exp Med. 2024 May 17;24(1):101. doi: 10.1007/s10238-024-01365-5. Clin Exp Med. 2024. PMID: 38758220 Free PMC article. Review.
-
Unraveling the glyco-immunity nexus in pancreatic cancer.Mol Cancer. 2025 Aug 4;24(1):211. doi: 10.1186/s12943-025-02417-4. Mol Cancer. 2025. PMID: 40759948 Free PMC article. Review.
-
Chimeric antibody targeting unique epitope on onco-mucin16 reduces tumor burden in pancreatic and lung malignancies.NPJ Precis Oncol. 2023 Aug 11;7(1):74. doi: 10.1038/s41698-023-00423-7. NPJ Precis Oncol. 2023. PMID: 37567918 Free PMC article.
-
Targeting Human Pancreatic Cancer with a Fluorophore-Conjugated Mucin 4 (MUC4) Antibody: Initial Characterization in Orthotopic Cell Line Mouse Models.J Clin Med. 2024 Oct 18;13(20):6211. doi: 10.3390/jcm13206211. J Clin Med. 2024. PMID: 39458160 Free PMC article.
References
-
- Puckett Y, Garfield K. Pancreatic Cancer. StatPearls. Treasure Island (FL): StatPearls Publishing.Copyright © 2021, StatPearls Publishing LLC.; 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

