Laboratory IR Spectra of the Ionic Oxidized Fullerenes C60O+ and C60OH
- PMID: 35533303
- PMCID: PMC9125688
- DOI: 10.1021/acs.jpca.2c01329
Laboratory IR Spectra of the Ionic Oxidized Fullerenes C60O+ and C60OH
Abstract
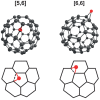
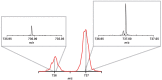
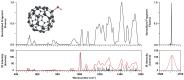
We present the first experimental vibrational spectra of gaseous oxidized derivatives of C60 in protonated and radical cation forms, obtained through infrared multiple-photon dissociation spectroscopy using the FELIX free-electron laser. Neutral C60O has two nearly iso-energetic isomers: the epoxide isomer in which the O atom bridges a CC bond that connects two six-membered rings and the annulene isomer in which the O atom inserts into a CC bond connecting a five- and a six-membered ring. To determine the isomer formed for C60O+ in our experiment─a question that cannot be confidently answered on the basis of the DFT-computed stabilities alone─we compare our experimental IR spectra to vibrational spectra predicted by DFT calculations. We conclude that the annulene-like isomer is formed in our experiment. For C60OH+, a strong OH stretch vibration observed in the 3 μm range of the spectrum immediately reveals its structure as C60 with a hydroxyl group attached, which is further confirmed by the spectrum in the 400-1600 cm-1 range. We compare the experimental spectra of C60O+ and C60OH+ to the astronomical IR emission spectrum of a fullerene-rich planetary nebula and discuss their astrophysical relevance.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
The influence of distribution of hydroxyl groups on vibrational spectra of fullerenol C60(OH)24 isomers: DFT study.Spectrochim Acta A Mol Biomol Spectrosc. 2015 Feb 5;136:1993-7. doi: 10.1016/j.saa.2014.08.023. Epub 2014 Aug 28. Spectrochim Acta A Mol Biomol Spectrosc. 2015. PMID: 25223813
-
Multifacets of Fullerene-Metal Clusters: From Fundamental to Application.Acc Chem Res. 2024 Jun 18;57(12):1670-1683. doi: 10.1021/acs.accounts.4c00130. Epub 2024 Apr 23. Acc Chem Res. 2024. PMID: 38654495
-
A structure-based analysis of the vibrational spectra of nitrosyl ligands in transition-metal coordination complexes and clusters.Spectrochim Acta A Mol Biomol Spectrosc. 2011 Jan;78(1):7-28. doi: 10.1016/j.saa.2010.08.001. Epub 2010 Aug 17. Spectrochim Acta A Mol Biomol Spectrosc. 2011. PMID: 21123107
-
Structure and energetics of C60O: a theoretical study.J Phys Chem A. 2010 Feb 4;114(4):1939-43. doi: 10.1021/jp9093386. J Phys Chem A. 2010. PMID: 20050638
-
Synthesis of a new class of fullerene derivative Li⁺@C₆₀O⁻(OH)₇ as a "cation-encapsulated anion nanoparticle".Nanoscale. 2013 Mar 21;5(6):2317-21. doi: 10.1039/c3nr33608e. Nanoscale. 2013. PMID: 23389385
Cited by
-
Structure and Vibrational Spectroscopy of C82 Fullerenol Valent Isomers: An Experimental and Theoretical Joint Study.Molecules. 2023 Feb 6;28(4):1569. doi: 10.3390/molecules28041569. Molecules. 2023. PMID: 36838557 Free PMC article.
-
Core-Hole Excitation Spectra of the Oxides and Hydrates of Fullerene C60 and Azafullerene C59N.Molecules. 2024 Jan 26;29(3):609. doi: 10.3390/molecules29030609. Molecules. 2024. PMID: 38338353 Free PMC article.
References
-
- Kroto H. W.; Heath J. R.; O’Brien S. C.; Curl R. F.; Smalley R. E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. 10.1038/318162a0. - DOI
-
- Acquah S. F. A.; Penkova A. V.; Markelov D. A.; Semisalova A. S.; Leonhardt B. E.; Magi J. M. Review—The Beautiful Molecule: 30 Years of C60 and Its Derivatives. ECS J. Solid State Sci. Technol. 2017, 6, M3155–M3162. 10.1149/2.0271706jss. - DOI
-
- von Helden G.; Holleman I.; Knippels G. M. H.; van der Meer A. F. G.; Meijer G. Infrared Resonance Enhanced Multiphoton Ionization of Fullerenes. Phys. Rev. Lett. 1997, 79, 5234–5237. 10.1103/PhysRevLett.79.5234. - DOI
LinkOut - more resources
Full Text Sources

