Age-Related Macular Degeneration Masquerade: A Review of Pentosan Polysulfate Maculopathy and Implications for Clinical Practice
- PMID: 35533330
- PMCID: PMC9096915
- DOI: 10.1097/APO.0000000000000504
Age-Related Macular Degeneration Masquerade: A Review of Pentosan Polysulfate Maculopathy and Implications for Clinical Practice
Abstract
Pentosan polysulfate (PPS) sodium (Elmiron) is the only Food and Drug Administration (FDA)-approved oral medication to treat interstitial cystitis, also known as bladder pain syndrome. A symptomatic pigmentary maculopathy associated with PPS was reported in 2018. Since then, recognition of this unique drug toxicity has increased rapidly. This potentially sight-threatening side effect prompted the FDA in June 2020 to update the label for PPS to warn about "retinal pigmentary changes." A challenging feature of pentosan maculopathy is its ability to mimic many other retinal conditions, including inherited retinal dystrophies such as pattern dystrophy, mitochondrially inherited diabetes and deafness, and Stargardt disease, and age-related macular degeneration. In this review, we discuss the history of PPS maculopathy and its implications for thousands of at-risk interstitial cystitis patients. We use published literature and an illustrative case from our institution to highlight the importance of diagnosing PPS maculopathy. We also compare PPS maculopathy to age-related macular degeneration, explain why differentiating between the 2 is clinically important, and highlight avenues for further research. Finally, we highlight the paucity of data on patients of color and why this lack of understanding may impact patient care.
Copyright © 2022 Asia-Pacific Academy of Ophthalmology. Published by Wolters Kluwer Health, Inc. on behalf of the Asia-Pacific Academy of Ophthalmology.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
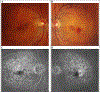

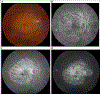
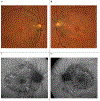
Similar articles
-
Association Between Pentosan Polysulfate Sodium and Retinal Disorders.JAMA Ophthalmol. 2022 Jan 1;140(1):37-42. doi: 10.1001/jamaophthalmol.2021.4778. JAMA Ophthalmol. 2022. PMID: 34792558 Free PMC article.
-
Pentosan polysulfate maculopathy.Surv Ophthalmol. 2022 Jan-Feb;67(1):83-96. doi: 10.1016/j.survophthal.2021.05.005. Epub 2021 May 14. Surv Ophthalmol. 2022. PMID: 34000253 Review.
-
Case Report: Pentosan Polysulfate Sodium Maculopathy Originally Diagnosed as Pattern Macular Dystrophy.Optom Vis Sci. 2021 Jun 1;98(6):552-556. doi: 10.1097/OPX.0000000000001702. Optom Vis Sci. 2021. PMID: 34039907
-
Pentosan polysulfate and a pigmentary maculopathy: causation versus correlation?Can J Urol. 2023 Dec;30(6):11732-11739. Can J Urol. 2023. PMID: 38104330 Review.
-
Expanded Clinical Spectrum of Pentosan Polysulfate Maculopathy: A Macula Society Collaborative Study.Ophthalmol Retina. 2022 Mar;6(3):219-227. doi: 10.1016/j.oret.2021.07.004. Epub 2021 Jul 21. Ophthalmol Retina. 2022. PMID: 34298229
Cited by
-
Fluorescence Lifetime Imaging of Human Retinal Pigment Epithelium in Pentosan Polysulfate Toxicity Using Adaptive Optics Scanning Light Ophthalmoscopy.Invest Ophthalmol Vis Sci. 2024 Apr 1;65(4):27. doi: 10.1167/iovs.65.4.27. Invest Ophthalmol Vis Sci. 2024. PMID: 38630675 Free PMC article.
-
Real-World Practices of Pentosan Polysulfate Maculopathy Screening in Various Clinical Settings and Practice-Associated Factors.J Clin Med. 2024 Aug 27;13(17):5090. doi: 10.3390/jcm13175090. J Clin Med. 2024. PMID: 39274302 Free PMC article.
-
Advanced pentosan polysulfate sodium maculopathy with low cumulative exposure and hydroxychloroquine use.Am J Ophthalmol Case Rep. 2024 Nov 22;36:102224. doi: 10.1016/j.ajoc.2024.102224. eCollection 2024 Dec. Am J Ophthalmol Case Rep. 2024. PMID: 39691632 Free PMC article.
References
-
- Sohn EH, Mullins RF, Stone EM. Ryan’s Retina. 6th ed. New York, US: Elsevier; 2018;44:953–996.

