Novel Regioselective Synthesis of Urolithin Glucuronides─Human Gut Microbiota Cometabolites of Ellagitannins and Ellagic Acid
- PMID: 35533350
- PMCID: PMC9121390
- DOI: 10.1021/acs.jafc.2c00170
Novel Regioselective Synthesis of Urolithin Glucuronides─Human Gut Microbiota Cometabolites of Ellagitannins and Ellagic Acid
Abstract
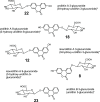
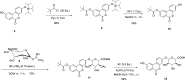
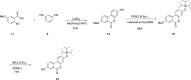
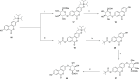
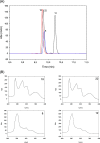
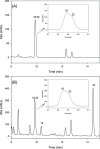
Urolithins (dibenzo-pyran-[b,d]-6 one derivatives) are human gut microbiota metabolites produced from the natural food antioxidant ellagic acid. Urolithins are better absorbed than ellagic acid and demonstrate biological activities that suggest that they are responsible for the health effects observed after consuming ellagitannin- and ellagic acid-containing foods. Urolithins occur in the systemic circulation as glucuronide conjugates following phase II metabolism. These phase II conjugates are essential for testing the urolithin mechanisms of action in human cell line bioassays. Urolithin glucuronides are not commercially available, and their biosynthesis leads to mixtures of regional isomers. This study describes a novel and regioselective synthesis of urolithin A (3,8-dihydroxy urolithin) 3- and 8-glucuronides and isourolithin A (3,9-dihydroxy urolithin) 3- and 9-glucuronides. The metabolites were characterized using 1H and 13C NMR spectroscopy and UV spectrophotometry. The presence of these metabolites in human subjects belonging to different urolithin metabotypes was also investigated.
Keywords: ellagic acid; glucuronides; gut microbiota metabolites; synthesis; urolithins.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Separation of Isomeric Forms of Urolithin Glucuronides Using Supercritical Fluid Chromatography.J Agric Food Chem. 2023 Feb 15;71(6):3033-3039. doi: 10.1021/acs.jafc.2c07145. Epub 2023 Jan 31. J Agric Food Chem. 2023. PMID: 36719954 Free PMC article.
-
Occurrence of urolithins, gut microbiota ellagic acid metabolites and proliferation markers expression response in the human prostate gland upon consumption of walnuts and pomegranate juice.Mol Nutr Food Res. 2010 Mar;54(3):311-22. doi: 10.1002/mnfr.200900152. Mol Nutr Food Res. 2010. PMID: 19885850 Clinical Trial.
-
Gastrointestinal stability of urolithins: an in vitro approach.Eur J Nutr. 2017 Feb;56(1):99-106. doi: 10.1007/s00394-015-1061-4. Epub 2015 Oct 6. Eur J Nutr. 2017. PMID: 26439723
-
Urolithins: a Comprehensive Update on their Metabolism, Bioactivity, and Associated Gut Microbiota.Mol Nutr Food Res. 2022 Nov;66(21):e2101019. doi: 10.1002/mnfr.202101019. Epub 2022 Feb 15. Mol Nutr Food Res. 2022. PMID: 35118817 Free PMC article. Review.
-
Urolithins, the rescue of "old" metabolites to understand a "new" concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status.Mol Nutr Food Res. 2017 Jan;61(1). doi: 10.1002/mnfr.201500901. Epub 2016 Jun 20. Mol Nutr Food Res. 2017. PMID: 27158799 Review.
Cited by
-
Urolithin A in Central Nervous System Disorders: Therapeutic Applications and Challenges.Biomedicines. 2025 Jun 25;13(7):1553. doi: 10.3390/biomedicines13071553. Biomedicines. 2025. PMID: 40722629 Free PMC article. Review.
-
Urolithin as a Metabolite of Ellagitannins and Ellagic Acid from Fruits and Nuts Produced by the Gut Microbiota: Its Role on Non-Communicable Diseases.Curr Nutr Rep. 2025 Apr 3;14(1):55. doi: 10.1007/s13668-025-00645-0. Curr Nutr Rep. 2025. PMID: 40180655 Review.
-
Chestnut Wood Mud as a Source of Ellagic Acid for Dermo-Cosmetic Applications.Antioxidants (Basel). 2022 Aug 28;11(9):1681. doi: 10.3390/antiox11091681. Antioxidants (Basel). 2022. PMID: 36139755 Free PMC article.
-
Separation of Isomeric Forms of Urolithin Glucuronides Using Supercritical Fluid Chromatography.J Agric Food Chem. 2023 Feb 15;71(6):3033-3039. doi: 10.1021/acs.jafc.2c07145. Epub 2023 Jan 31. J Agric Food Chem. 2023. PMID: 36719954 Free PMC article.
References
-
- Cerdá B.; Espín J. C.; Parra S.; Martínez P.; Tomás-Barberán F. A. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora in healthy humans. Eur. J. Nutr. 2004, 43, 205–220. 10.1007/s00394-004-0461-7. - DOI - PubMed
-
- Tomás-Barberán F. A.; González-Sarrías A.; García-Villalba R.; Nuñez-Sanchez M. A.; Selma M. V.; García-Conesa M. T.; Espin J. C. Urolithins, the rescue of ‘old’ metabolites to understand a ‘new’ concept; Metabotypes as a nexus between phenolic metabolism, microbiota dysbiosis and host health status. Mol. Nutr. Food Res. 2017, 61, 150090110.1002/mnfr.201500901. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

