Medullary astrocytes mediate irregular breathing patterns generation in chronic heart failure through purinergic P2X7 receptor signalling
- PMID: 35533501
- PMCID: PMC9097632
- DOI: 10.1016/j.ebiom.2022.104044
Medullary astrocytes mediate irregular breathing patterns generation in chronic heart failure through purinergic P2X7 receptor signalling
Abstract
Background: Breathing disorders (BD) (apnoeas/hypopneas, periodic breathing) are highly prevalent in chronic heart failure (CHF) and are associated with altered central respiratory control. Ample evidence identifies the retrotrapezoid nucleus (RTN) as an important chemosensitivity region for ventilatory control and generation of BD in CHF, however little is known about the cellular mechanisms underlying the RTN/BD relationship. Within the RTN, astrocyte-mediated purinergic signalling modulates respiration, but the potential contribution of RTN astrocytes to BD in CHF has not been explored.
Methods: Selective neuron and/or astrocyte-targeted interventions using either optogenetic and chemogenetic manipulations in the RTN of CHF rats were used to unveil the contribution of the RTN on the development/maintenance of BD, the role played by astrocytes in BD and the molecular mechanism underpinning these alterations.
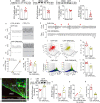
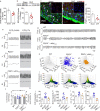
Findings: We showed that episodic photo-stimulation of RTN neurons triggered BD in healthy rats, and that RTN neurons ablation in CHF animals eliminates BD. Also, we found a reduction in astrocytes activity and ATP bioavailability within the RTN of CHF rats, and that chemogenetic restoration of normal RTN astrocyte activity and ATP levels improved breathing regularity in CHF. Importantly, P"X/ P2X7 receptor (P2X7r) expression was reduced in RTN astrocytes from CHF rats and viral vector-mediated delivery of human P2X7 P2X7r into astrocytes increases ATP bioavailability and abolished BD.
Interpretation: Our results support that RTN astrocytes play a pivotal role on BD generation and maintenance in the setting CHF by a mechanism encompassing P2X7r signalling.
Funding: This study was funded by the National Research and Development Agency of Chile (ANID).
Keywords: Astrocyte; Chronic heart failure; Disordered breathing; P2X7 receptor; Retrotrapezoid nucleus.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures




References
-
- Parati G., Lombardi C., Castagna F., et al. Heart failure and sleep disorders. Nat Rev Cardiol. 2016;13(7):389–403. - PubMed
-
- Giannoni A., Emdin M., Bramanti F., et al. Combined increased chemosensitivity to hypoxia and hypercapnia as a prognosticator in heart failure. J Am Coll Cardiol. 2009;53(21):1975–1980. - PubMed
-
- Narkiewicz K., Pesek C.A., van de Borne P.J., Kato M., Somers V.K. Enhanced sympathetic and ventilatory responses to central chemoreflex activation in heart failure. Circulation. 1999;100(3):262–267. - PubMed
-
- Hanly P., Zuberi N., Gray R. Pathogenesis of Cheyne-Stokes respiration in patients with congestive heart failure. Relationship to arterial PCO2. Chest. 1993;104(4):1079–1084. - PubMed
-
- Nogues M.A., Benarroch E. Abnormalities of respiratory control and the respiratory motor unit. Neurologist. 2008;14(5):273–288. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

