Partial and complete loss of myosin binding protein H-like cause cardiac conduction defects
- PMID: 35533732
- PMCID: PMC9329245
- DOI: 10.1016/j.yjmcc.2022.04.012
Partial and complete loss of myosin binding protein H-like cause cardiac conduction defects
Abstract
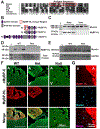
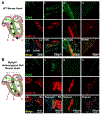
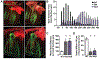
A premature truncation of MYBPHL in humans and a loss of Mybphl in mice is associated with dilated cardiomyopathy, atrial and ventricular arrhythmias, and atrial enlargement. MYBPHL encodes myosin binding protein H-like (MyBP-HL). Prior work in mice indirectly identified Mybphl expression in the atria and in small puncta throughout the ventricle. Because of its genetic association with human and mouse cardiac conduction system disease, we evaluated the anatomical localization of MyBP-HL and the consequences of loss of MyBP-HL on conduction system function. Immunofluorescence microscopy of normal adult mouse ventricles identified MyBP-HL-positive ventricular cardiomyocytes that co-localized with the ventricular conduction system marker contactin-2 near the atrioventricular node and in a subset of Purkinje fibers. Mybphl heterozygous ventricles had a marked reduction of MyBP-HL-positive cells compared to controls. Lightsheet microscopy of normal perinatal day 5 mouse hearts showed enrichment of MyBP-HL-positive cells within and immediately adjacent to the contactin-2-positive ventricular conduction system, but this association was not apparent in Mybphl heterozygous hearts. Surface telemetry of Mybphl-null mice revealed atrioventricular block and atrial bigeminy, while intracardiac pacing revealed a shorter atrial relative refractory period and atrial tachycardia. Calcium transient analysis of isolated Mybphl-null atrial cardiomyocytes demonstrated an increased heterogeneity of calcium release and faster rates of calcium release compared to wild type controls. Super-resolution microscopy of Mybphl heterozygous and homozygous null atrial cardiomyocytes showed ryanodine receptor disorganization compared to wild type controls. Abnormal calcium release, shorter atrial refractory period, and atrial dilation seen in Mybphl null, but not wild type control hearts, agree with the observed atrial arrhythmias, bigeminy, and atrial tachycardia, whereas the proximity of MyBP-HL-positive cells with the ventricular conduction system provides insight into how a predominantly atrial expressed gene contributes to ventricular arrhythmias and ventricular dysfunction.
Keywords: Atrial cardiomyocyte; Cardiomyopathy; MYBPHL; MyBP-HL; Myosin binding protein; Ventricular conduction system.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures




References
-
- Barefield DY, Puckelwartz MJ, Kim EY, Wilsbacher LD, Vo AH, Waters EA, Earley JU, Hadhazy M, Dellefave-Castillo L, Pesce LL, McNally EM, Experimental Modeling Supports a Role for MyBP-HL as a Novel Myofilament Component in Arrhythmia and Dilated Cardiomyopathy, Circulation 136(16) (2017) 1477–1491. - PMC - PubMed
-
- Alyonycheva T, Cohen-Gould L, Siewert C, Fischman DA, Mikawa T, Skeletal muscle-specific myosin binding protein-H is expressed in Purkinje fibers of the cardiac conduction system, Circ Res 80(5) (1997) 665–72. - PubMed
-
- Mouton J, Loos B, Moolman-Smook JC, Kinnear CJ, Ascribing novel functions to the sarcomeric protein, myosin binding protein H (MyBPH) in cardiac sarcomere contraction, Exp Cell Res 331(2) (2015) 338–51. - PubMed
-
- Mouton JM, van der Merwe L, Goosen A, Revera M, Brink PA, Moolman-Smook JC, Kinnear C, MYBPH acts as modifier of cardiac hypertrophy in hypertrophic cardiomyopathy (HCM) patients, Hum Genet 135(5) (2016) 477–483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

