Solid organ transplantation from donors with recent or current SARS-CoV-2 infection: A systematic review
- PMID: 35533977
- PMCID: PMC9074299
- DOI: 10.1016/j.accpm.2022.101098
Solid organ transplantation from donors with recent or current SARS-CoV-2 infection: A systematic review
Abstract
Background: Solid-organ transplantation (SOT) from SARS-CoV-2 positive donors could be a life-saving opportunity worth grasping. We perform a systematic review to evaluate the recipient outcomes of SOT from donors with recent or current SARS-CoV-2 infection.
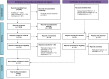
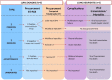
Methods: Search strategy was performed in PubMed, Cochrane COVID-19 Study Register, and Web of Science databases from the 1st of January 2019 to the 31st of December 2021. SOT adult recipients from a donor with past or current SARS-CoV-2 infection were elegible for inclusion. Outcomes were viral transmission, COVID-19 symptoms, mortality, hospital stay, and complications. PROSPERO Register Number: CRD42022303242 FINDINGS: Sixty-nine recipients received 48 kidneys, 18 livers and 3 hearts from 57 donors. Six additional transplants from positive lungs were identified. IgG+ anti-SARS-CoV-2 titers were detected among 10/16 recipients; only 4% (3/69) recipients were vaccinated. Non-lung transplant recipients received organs from 10/57 (17.5%) donors with persistent COVID-19. In 18/57 donors, SARS-CoV-2 RNA was detected (median 32 Cycle threshold [Ct]) at procurement. Among non-lung transplant recipients, SARS-CoV-2 viral transmission was not documented. Four patients presented delayed graft dysfunction, two patients acute rejection, and two patients died of septic shock. The median (IQR) hospital stay was 18 (11-28) days in recipients from symptomatic donors. Viral transmission occurred from three lung donors to their recipients, who developed COVID-19 symptoms. One of the recipients subsequently died.
Conclusion: Use of non-lung (kidney, liver and heart) organs from SARS-CoV-2 positive donors seem to be a safe practice, with a low risk of transmission irrespective of the presence of symptoms at the time of procurement. Low viral replication (Ct > 30) was safe among non-lung donors, even if persistently symptomatic at procurement.
Keywords: COVID-19; Organ donation; Solid organ transplantation; Viral transmission.
Copyright © 2022 Société française d'anesthésie et de réanimation (Sfar). Published by Elsevier Masson SAS. All rights reserved.
Figures
Comment in
-
Organ donation and COVID-19: Should precautionary principle still apply?Anaesth Crit Care Pain Med. 2022 Aug;41(4):101120. doi: 10.1016/j.accpm.2022.101120. Epub 2022 Jun 30. Anaesth Crit Care Pain Med. 2022. PMID: 35779803 Free PMC article. No abstract available.
References
-
- Organ Procurement and Transplantation Network (OPTN) COVID-19. Organ procurement and transplantation network. 2022. https://optn.transplant.hrsa.gov/covid-19 (Accessed 21 March 2022)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous