Retinol-binding protein 2 (RBP2): More than just dietary retinoid uptake
- PMID: 35533980
- PMCID: PMC9191623
- DOI: 10.1016/j.bbalip.2022.159179
Retinol-binding protein 2 (RBP2): More than just dietary retinoid uptake
Abstract
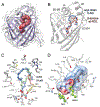
Retinol-binding protein 2 (RBP2, also known as cellular retinol-binding protein 2 (CRBP2)) is a member of the fatty acid-binding protein family and has been extensively studied for its role in facilitating dietary vitamin A (retinol) uptake and metabolism within enterocytes of the small intestine. RBP2 is present in highest concentrations in the proximal small intestine where it constitutes approximately 0.1-0.5% of soluble protein. Recent reports have established that RBP2 binds monoacylglycerols (MAGs) with high affinity, including the canonical endocannabinoid 2-arachidonoylglycerol (2-AG). Crystallographic studies reveal that retinol, 2-AG, or other long-chain MAGs alternatively can bind in the retinol-binding pocket of RBP2. It also has been demonstrated recently that Rbp2-deficient mice are more susceptible to developing obesity and associated metabolic phenotypes when exposed to a high fat diet, or as they age when fed a conventional chow diet. When subjected to an oral fat challenge, the Rbp2-deficient mice release into the circulation significantly more, compared to littermate controls, of the intestinal hormone glucose-dependent insulinotropic polypeptide (GIP). These new findings regarding RBP2 structure and actions within the intestine are the focus of this review.
Keywords: 2-monoacylglycerol; All-trans-retinoic acid; CRBP2; Cellular retinol-binding protein 2; Glucose-dependent insulinotropic polypeptide (GIP); Incretin; Obesity; RBP2; Retinoids; Retinol-binding proteins; Vitamin A.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest
None of the authors of this review article have any conflicts of interest that have influenced the writing of the review.
Figures

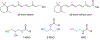

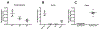
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

