Notch Signaling in Vascular Endothelial and Mural Cell Communications
- PMID: 35534207
- PMCID: PMC9435572
- DOI: 10.1101/cshperspect.a041159
Notch Signaling in Vascular Endothelial and Mural Cell Communications
Abstract
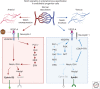
The Notch signaling pathway is a highly versatile and evolutionarily conserved mechanism with an important role in cell fate determination. Notch signaling plays a vital role in vascular development, regulating several fundamental processes such as angiogenesis, arterial/venous differentiation, and mural cell investment. Aberrant Notch signaling can result in severe vascular phenotypes as observed in cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) and Alagille syndrome. It is known that vascular endothelial cells and mural cells interact to regulate vessel formation, cell maturation, and stability of the vascular network. Defective endothelial-mural cell interactions are a common phenotype in diseases characterized by impaired vascular integrity. Further refinement of the role of Notch signaling in the vascular junctions will be critical to attempts to modulate Notch in the context of human vascular disease. In this review, we aim to consolidate and summarize our current understanding of Notch signaling in the vascular endothelial and mural cells during development and in the adult vasculature.
Copyright © 2022 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures



Similar articles
-
Notch Signaling in Vascular Smooth Muscle Cells.Adv Pharmacol. 2017;78:351-382. doi: 10.1016/bs.apha.2016.07.002. Epub 2016 Aug 26. Adv Pharmacol. 2017. PMID: 28212801 Free PMC article. Review.
-
Regulation of vascular morphogenesis by Notch signaling.Genes Dev. 2007 Oct 15;21(20):2511-24. doi: 10.1101/gad.1589207. Genes Dev. 2007. PMID: 17938237 Review.
-
Notch signaling in the vasculature.Curr Top Dev Biol. 2010;92:277-309. doi: 10.1016/S0070-2153(10)92009-7. Curr Top Dev Biol. 2010. PMID: 20816399 Free PMC article. Review.
-
Notch signalling in smooth muscle cells during development and disease.Cardiovasc Res. 2012 Jul 15;95(2):138-46. doi: 10.1093/cvr/cvs019. Epub 2012 Jan 19. Cardiovasc Res. 2012. PMID: 22266753 Review.
-
Notch and vascular smooth muscle cell phenotype.Circ Res. 2008 Dec 5;103(12):1370-82. doi: 10.1161/CIRCRESAHA.108.187534. Circ Res. 2008. PMID: 19059839 Review.
Cited by
-
A perspective on Notch signalling in progression and arrhythmogenesis in familial hypertrophic and dilated cardiomyopathies.Philos Trans R Soc Lond B Biol Sci. 2023 Jun 19;378(1879):20220176. doi: 10.1098/rstb.2022.0176. Epub 2023 May 1. Philos Trans R Soc Lond B Biol Sci. 2023. PMID: 37122209 Free PMC article. Review.
-
Blood vessel organoids generated by base editing and harboring single nucleotide variation in Notch3 effectively recapitulate CADASIL-related pathogenesis.Mol Neurobiol. 2024 Nov;61(11):9171-9183. doi: 10.1007/s12035-024-04141-4. Epub 2024 Apr 9. Mol Neurobiol. 2024. PMID: 38592587 Free PMC article.
-
Progress to Clarify How NOTCH3 Mutations Lead to CADASIL, a Hereditary Cerebral Small Vessel Disease.Biomolecules. 2024 Jan 18;14(1):127. doi: 10.3390/biom14010127. Biomolecules. 2024. PMID: 38254727 Free PMC article. Review.
-
Downregulation of Notch Signaling-Stimulated Genes in Neurovascular Unit Alterations Induced by Chronic Cerebral Hypoperfusion.Immun Inflamm Dis. 2024 Nov;12(11):e70082. doi: 10.1002/iid3.70082. Immun Inflamm Dis. 2024. PMID: 39607309 Free PMC article.
-
Biology of vascular mural cells.Development. 2023 Aug 15;150(16):dev200271. doi: 10.1242/dev.200271. Epub 2023 Aug 28. Development. 2023. PMID: 37642459 Free PMC article. Review.
References
-
- Arboleda-Velasquez JF, Manent J, Lee JH, Tikka S, Ospina C, Vanderburg CR, Frosch MP, Rodriguez-Falcon M, Villen J, Gygi S, et al. 2011. Hypomorphic Notch 3 alleles link Notch signaling to ischemic cerebral small-vessel disease. Proc Natl Acad Sci 108: E128–E135. 10.1073/pnas.1101964108 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
