Buttons and Zippers: Endothelial Junctions in Lymphatic Vessels
- PMID: 35534209
- PMCID: PMC9643678
- DOI: 10.1101/cshperspect.a041178
Buttons and Zippers: Endothelial Junctions in Lymphatic Vessels
Abstract
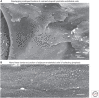
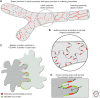
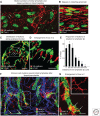
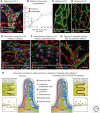
Button-like junctions are discontinuous contacts at the border of oak-leaf-shaped endothelial cells of initial lymphatic vessels. These junctions are distinctively different from continuous zipper-like junctions that create the endothelial barrier in collecting lymphatics and blood vessels. Button junctions are point contacts, spaced about 3 µm apart, that border valve-like openings where fluid and immune cells enter lymphatics. In intestinal villi, openings between button junctions in lacteals also serve as entry routes for chylomicrons. Like zipper junctions that join endothelial cells, buttons consist of adherens junction proteins (VE-cadherin) and tight junction proteins (claudin-5, occludin, and others). Buttons in lymphatics form from zipper junctions during embryonic development, can convert into zippers in disease or after experimental genetic or pharmacological manipulation, and can revert back to buttons with treatment. Multiple signaling pathways and local microenvironmental factors have been found to contribute to button junction plasticity and could serve as therapeutic targets in pathological conditions ranging from pulmonary edema to obesity.
Copyright © 2022 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


Similar articles
-
Functionally specialized junctions between endothelial cells of lymphatic vessels.J Exp Med. 2007 Oct 1;204(10):2349-62. doi: 10.1084/jem.20062596. Epub 2007 Sep 10. J Exp Med. 2007. PMID: 17846148 Free PMC article.
-
Plasticity of button-like junctions in the endothelium of airway lymphatics in development and inflammation.Am J Pathol. 2012 Jun;180(6):2561-75. doi: 10.1016/j.ajpath.2012.02.019. Epub 2012 Apr 23. Am J Pathol. 2012. PMID: 22538088 Free PMC article.
-
Anatomy and pathology of lymphatic vessels under physiological and inflammatory conditions in the mouse diaphragm.Microvasc Res. 2023 Jan;145:104438. doi: 10.1016/j.mvr.2022.104438. Epub 2022 Sep 16. Microvasc Res. 2023. PMID: 36122645
-
MicroRNA Regulation of Endothelial Junction Proteins and Clinical Consequence.Mediators Inflamm. 2016;2016:5078627. doi: 10.1155/2016/5078627. Epub 2016 Nov 24. Mediators Inflamm. 2016. PMID: 27999452 Free PMC article. Review.
-
Endothelial and virgultar cell formations in the mammalian lymph node sinus: endothelial differentiation morphotypes characterized by a special kind of junction (complexus adhaerens).Cell Tissue Res. 2009 Jan;335(1):109-41. doi: 10.1007/s00441-008-0700-y. Epub 2008 Nov 18. Cell Tissue Res. 2009. PMID: 19015886 Review.
Cited by
-
Collecting lymphatics unzip to drain injured lungs.Nat Cardiovasc Res. 2025 Aug;4(8):959-961. doi: 10.1038/s44161-025-00682-6. Nat Cardiovasc Res. 2025. PMID: 40676262 No abstract available.
-
Ameliorating lipopolysaccharide induced acute lung injury with Lianhua Qingke: focus on pulmonary endothelial barrier protection.J Thorac Dis. 2024 Oct 31;16(10):6899-6917. doi: 10.21037/jtd-24-700. Epub 2024 Oct 29. J Thorac Dis. 2024. PMID: 39552861 Free PMC article.
-
The Lymphatic Vascular System in Extracellular Vesicle-Mediated Tumor Progression.Cancers (Basel). 2024 Dec 2;16(23):4039. doi: 10.3390/cancers16234039. Cancers (Basel). 2024. PMID: 39682225 Free PMC article. Review.
-
Intestinal Lymphatic Dysfunction in Kidney Disease.Circ Res. 2023 Apr 28;132(9):1226-1245. doi: 10.1161/CIRCRESAHA.122.321671. Epub 2023 Apr 27. Circ Res. 2023. PMID: 37104557 Free PMC article. Review.
-
Characterization of Photo-Crosslinked Methacrylated Type I Collagen as a Platform to Investigate the Lymphatic Endothelial Cell Response.Lymphatics. 2024 Sep;2(3):177-194. doi: 10.3390/lymphatics2030015. Epub 2024 Sep 19. Lymphatics. 2024. PMID: 39664172 Free PMC article.
References
-
- Arasa J, Collado-Diaz V, Kritikos I, Medina-Sanchez JD, Friess MC, Sigmund EC, Schineis P, Hunter MC, Tacconi C, Paterson N, et al. 2021b. Upregulation of VCAM-1 in lymphatic collectors supports dendritic cell entry and rapid migration to lymph nodes in inflammation. J Exp Med 218: 1–18. 10.1084/jem.20201413 - DOI - PMC - PubMed
-
- Aselli G. 1627. De lactibus sive lacteis venis, quarto vasorum mesarai corum genere novo invento. G.B. Bidelli, Milan, Italy.
