Neuropilin 2/Plexin-A3 Receptors Regulate the Functional Connectivity and the Excitability in the Layers 4 and 5 of the Cerebral Cortex
- PMID: 35534225
- PMCID: PMC9188426
- DOI: 10.1523/JNEUROSCI.1965-21.2022
Neuropilin 2/Plexin-A3 Receptors Regulate the Functional Connectivity and the Excitability in the Layers 4 and 5 of the Cerebral Cortex
Abstract
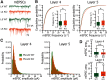
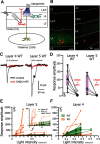
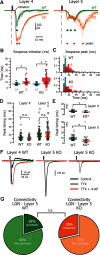
The functions of cortical networks are progressively established during development by series of events shaping the neuronal connectivity. Synaptic elimination, which consists of removing the supernumerary connections generated during the earlier stages of cortical development, is one of the latest stages in neuronal network maturation. The semaphorin 3F coreceptors neuropilin 2 (Nrp2) and plexin-A3 (PlxnA3) may play an important role in the functional maturation of the cerebral cortex by regulating the excess dendritic spines on cortical excitatory neurons. Yet, the identity of the connections eliminated under the control of Nrp2/PlxnA3 signaling is debated, and the importance of this synaptic refinement for cortical functions remains poorly understood. Here, we show that Nrp2/PlxnA3 controls the spine densities in layer 4 (L4) and on the apical dendrite of L5 neurons of the sensory and motor cortices. Using a combination of neuroanatomical, ex vivo electrophysiology, and in vivo functional imaging techniques in Nrp2 and PlxnA3 KO mice of both sexes, we disprove the hypothesis that Nrp2/PlxnA3 signaling is required to maintain the ectopic thalamocortical connections observed during embryonic development. We also show that the absence of Nrp2/PlxnA3 signaling leads to the hyperexcitability and excessive synchronization of the neuronal activity in L5 and L4 neuronal networks, suggesting that this system could participate in the refinement of the recurrent corticocortical connectivity in those layers. Altogether, our results argue for a role of semaphorin-Nrp2/PlxnA3 signaling in the proper maturation and functional connectivity of the cerebral cortex, likely by controlling the refinement of recurrent corticocortical connections.SIGNIFICANCE STATEMENT The function of a neuronal circuit is mainly determined by the connections that neurons establish with one another during development. Understanding the mechanisms underlying the establishment of the functional connectivity is fundamental to comprehend how network functions are implemented, and to design treatments aiming at restoring damaged neuronal circuits. Here, we show that the cell surface receptors for the family of semaphorin guidance cues neuropilin 2 (Nrp2) and plexin-A3 (PlxnA3) play an important role in shaping the functional connectivity of the cerebral cortex likely by trimming the recurrent connections in layers 4 and 5. By removing the supernumerary inputs generated during early development, Nrp2/PlxnA3 signaling reduces the neuronal excitability and participates in the maturation of the cortical network functions.
Keywords: cortex maturation; cortical layers; functional connectivity; sema 3F signaling; synaptic pruning; thalamocortical.
Copyright © 2022 the authors.
Figures




Similar articles
-
Neuropilin 2 Signaling Mediates Corticostriatal Transmission, Spine Maintenance, and Goal-Directed Learning in Mice.J Neurosci. 2019 Nov 6;39(45):8845-8859. doi: 10.1523/JNEUROSCI.1006-19.2019. Epub 2019 Sep 20. J Neurosci. 2019. PMID: 31541021 Free PMC article.
-
Close Homolog of L1 Regulates Dendritic Spine Density in the Mouse Cerebral Cortex Through Semaphorin 3B.J Neurosci. 2019 Aug 7;39(32):6233-6250. doi: 10.1523/JNEUROSCI.2984-18.2019. Epub 2019 Jun 10. J Neurosci. 2019. PMID: 31182634 Free PMC article.
-
Palmitoylation regulates neuropilin-2 localization and function in cortical neurons and conveys specificity to semaphorin signaling via palmitoyl acyltransferases.Elife. 2023 Apr 3;12:e83217. doi: 10.7554/eLife.83217. Elife. 2023. PMID: 37010951 Free PMC article.
-
Discovery of semaphorin receptors, neuropilin and plexin, and their functions in neural development.J Neurobiol. 2004 Apr;59(1):24-33. doi: 10.1002/neu.10337. J Neurobiol. 2004. PMID: 15007824 Review.
-
Axon pruning and synaptic development: how are they per-plexin?Neuroscientist. 2006 Oct;12(5):398-409. doi: 10.1177/1073858406292631. Neuroscientist. 2006. PMID: 16957002 Review.
Cited by
-
Multiplexed CRISPRi Reveals a Transcriptional Switch Between KLF Activators and Repressors in the Maturing Neocortex.bioRxiv [Preprint]. 2025 Feb 15:2025.02.07.636951. doi: 10.1101/2025.02.07.636951. bioRxiv. 2025. PMID: 39975013 Free PMC article. Preprint.
-
Transcriptomic changes across subregions of the primate cerebellum support the evolution of uniquely human behaviors.bioRxiv [Preprint]. 2025 Mar 3:2025.03.03.641249. doi: 10.1101/2025.03.03.641249. bioRxiv. 2025. PMID: 40093170 Free PMC article. Preprint.
References
-
- Brainard DH (1997) The Psychophysics Toolbox. Spat Vis 10:433–436. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
