Motor Impairments and Dopaminergic Defects Caused by Loss of Leucine-Rich Repeat Kinase Function in Mice
- PMID: 35534227
- PMCID: PMC9186805
- DOI: 10.1523/JNEUROSCI.0140-22.2022
Motor Impairments and Dopaminergic Defects Caused by Loss of Leucine-Rich Repeat Kinase Function in Mice
Abstract
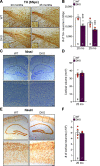
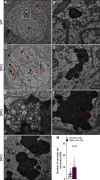
Mutations in leucine-rich repeat kinase 2 (LRRK2) are the most common genetic cause of Parkinson's disease (PD), but the pathogenic mechanism underlying LRRK2 mutations remains unresolved. In this study, we investigate the consequence of inactivation of LRRK2 and its functional homolog LRRK1 in male and female mice up to 25 months of age using behavioral, neurochemical, neuropathological, and ultrastructural analyses. We report that LRRK1 and LRRK2 double knock-out (LRRK DKO) mice exhibit impaired motor coordination at 12 months of age before the onset of dopaminergic neuron loss in the substantia nigra (SNpc). Moreover, LRRK DKO mice develop age-dependent, progressive loss of dopaminergic terminals in the striatum. Evoked dopamine (DA) release measured by fast-scan cyclic voltammetry in the dorsal striatum is also reduced in the absence of LRRK. Furthermore, LRRK DKO mice at 20-25 months of age show substantial loss of dopaminergic neurons in the SNpc. The surviving SNpc neurons in LRRK DKO mice at 25 months of age accumulate large numbers of autophagic and autolysosomal vacuoles and are accompanied with microgliosis. Surprisingly, the cerebral cortex is unaffected, as shown by normal cortical volume and neuron number as well as unchanged number of apoptotic cells and microglia in LRRK DKO mice at 25 months. These findings show that loss of LRRK function causes impairments in motor coordination, degeneration of dopaminergic terminals, reduction of evoked DA release, and selective loss of dopaminergic neurons in the SNpc, indicating that LRRK DKO mice are unique models for better understanding dopaminergic neurodegeneration in PD.SIGNIFICANCE STATEMENT Our current study employs a genetic approach to uncover the normal function of the LRRK family in the brain during mouse life span. Our multidisciplinary analysis demonstrates a critical normal physiological role of LRRK in maintaining the integrity and function of dopaminergic terminals and neurons in the aging brain, and show that LRRK DKO mice recapitulate several key features of PD and provide unique mouse models for elucidating molecular mechanisms underlying dopaminergic neurodegeneration in PD.
Keywords: LRRK2; Parkinson's disease; SNpc; dopamine release; knock-out mice; striatum.
Copyright © 2022 the authors.
Figures





Similar articles
-
Cell-autonomous role of leucine-rich repeat kinase in the protection of dopaminergic neuron survival.Elife. 2024 Jun 10;12:RP92673. doi: 10.7554/eLife.92673. Elife. 2024. PMID: 38856715 Free PMC article.
-
Cell autonomous role of leucine-rich repeat kinase in protection of dopaminergic neuron survival.bioRxiv [Preprint]. 2024 Mar 7:2023.10.06.561293. doi: 10.1101/2023.10.06.561293. bioRxiv. 2024. Update in: Elife. 2024 Jun 10;12:RP92673. doi: 10.7554/eLife.92673. PMID: 37873418 Free PMC article. Updated. Preprint.
-
Age-Dependent Dopaminergic Neurodegeneration and Impairment of the Autophagy-Lysosomal Pathway in LRRK-Deficient Mice.Neuron. 2017 Nov 15;96(4):796-807.e6. doi: 10.1016/j.neuron.2017.09.036. Epub 2017 Oct 19. Neuron. 2017. PMID: 29056298 Free PMC article.
-
LRRK 2 gene mutations in the pathophysiology of the ROCO domain and therapeutic targets for Parkinson's disease: a review.J Biomed Sci. 2018 Jun 14;25(1):52. doi: 10.1186/s12929-018-0454-0. J Biomed Sci. 2018. PMID: 29903014 Free PMC article. Review.
-
Genetic analysis of Parkinson's disease-linked leucine-rich repeat kinase 2.Biochem Soc Trans. 2012 Oct;40(5):1042-6. doi: 10.1042/BST20120112. Biochem Soc Trans. 2012. PMID: 22988862 Free PMC article. Review.
Cited by
-
Molecular Pathways Involved in LRRK2-Linked Parkinson's Disease: A Systematic Review.Int J Mol Sci. 2022 Oct 3;23(19):11744. doi: 10.3390/ijms231911744. Int J Mol Sci. 2022. PMID: 36233046 Free PMC article.
-
Human Presenilin-1 delivered by AAV9 rescues impaired γ-secretase activity, memory deficits, and neurodegeneration in Psen mutant mice.Proc Natl Acad Sci U S A. 2023 Oct 17;120(42):e2306714120. doi: 10.1073/pnas.2306714120. Epub 2023 Oct 10. Proc Natl Acad Sci U S A. 2023. PMID: 37816062 Free PMC article.
-
Roles of LRRK2 and its orthologs in protecting against neurodegeneration and neurodevelopmental defects.Front Cell Dev Biol. 2025 Apr 30;13:1569733. doi: 10.3389/fcell.2025.1569733. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40371391 Free PMC article.
-
Cell-autonomous role of leucine-rich repeat kinase in the protection of dopaminergic neuron survival.Elife. 2024 Jun 10;12:RP92673. doi: 10.7554/eLife.92673. Elife. 2024. PMID: 38856715 Free PMC article.
References
-
- Di Fonzo A, Rohé CF, Ferreira J, Chien HF, Vacca L, Stocchi F, Guedes L, Fabrizio E, Manfredi M, Vanacore N, Goldwurm S, Breedveld G, Sampaio C, Meco G, Barbosa E, Oostra BA, Bonifati V (2005) A frequent LRRK2 gene mutation associated with autosomal dominant Parkinson's disease. Lancet 365:412–415. 10.1016/S0140-6736(05)17829-5 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
