Association of Anti-Rotavirus IgA Seroconversion with Growth, Environmental Enteric Dysfunction and Enteropathogens in Rural Pakistani Infants
- PMID: 35534310
- PMCID: PMC9168439
- DOI: 10.1016/j.vaccine.2022.04.032
Association of Anti-Rotavirus IgA Seroconversion with Growth, Environmental Enteric Dysfunction and Enteropathogens in Rural Pakistani Infants
Abstract
Background: The underperformance of oral vaccines in children of low- and middle-income countries is partly attributable to underlying environmental enteric dysfunction (EED).
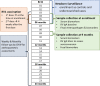
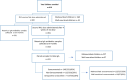
Methodology: We conducted a longitudinal, community-based study to evaluate the association of oral rotavirus vaccine (Rotarix®) seroconversion with growth anthropometrics, EED biomarkers and intestinal enteropathogens in Pakistani infants. Children were enrolled between three to six months of their age based on their nutritional status. We measured serum anti-rotavirus immunoglobulin A (IgA) at enrollment and nine months of age with EED biomarkers and intestinal enteropathogens.
Results: A total of 391 infants received two doses of rotavirus (RV) vaccine. 331/391 provided paired blood samples. Of these 331 children, 45% seroconverted at 9 months of age, 35% did not seroconvert and 20% were seropositive at baseline. Non-seroconverted children were more likely to be stunted, wasted and underweight at enrollment. In univariate analysis, insulin-like growth factor (IGF) concentration at 6 months were higher in seroconverters, median (25th, 75th percentile): 26.3 (16.5, 43.5) ng/ml vs. 22.5 (13.6, 36.3) ng/ml for non-seroconverters, p-value = 0.024. At nine months, fecal myeloperoxidase (MPO) concentrations were significantly lower in seroconverters, 3050(1250, 7587) ng/ml vs. 4623.3 (2189, 11650) ng/ml in non-seroconverted children, p-value = 0.017. In multivariable logistic regression analysis, alpha-1 acid glycoprotein (AGP) and IGF-1 concentrations were positively associated with seroconversion at six months. The presence of sapovirus and rotavirus in fecal samples at the time of rotavirus administration, was associated with non-seroconversion and seroconversion, respectively.
Conclusion: We detected high baseline RV seropositivity and impaired RV vaccine immunogenicity in this high-risk group of children. Healthy growth, serum IGF-1 and AGP, and fecal shedding of rotavirus were positively associated with RV IgA seroconversion following immunization, whereas the presence of sapovirus was more common in non-seroconverters.
Trial registration: Clinical Trials ID: NCT03588013.
Keywords: Biomarkers; Environmental enteric dysfunction; Infants; Malnutrition; Oral vaccines; Rotavirus vaccine; Stunting.
Copyright © 2022 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Solomons N.W. Environmental contamination and chronic inflammation influence human growth potential. J. nutr. 2003;133(5):1237. - PubMed
-
- Kelly P., Menzies I., Crane R., Zulu I., Nickols C., Feakins R., Mwansa J., Mudenda V., Katubulushi M., Greenwald S., Farthing M. Responses of small intestinal architecture and function over time to environmental factors in a tropical population. Am J Trop Med Hyg. 2004;70(4):412–419. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

