Hydrostatic pressure as a driver of cell and tissue morphogenesis
- PMID: 35534334
- PMCID: PMC9529827
- DOI: 10.1016/j.semcdb.2022.04.021
Hydrostatic pressure as a driver of cell and tissue morphogenesis
Abstract
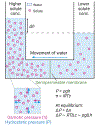
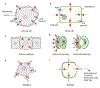
Morphogenesis, the process by which tissues develop into functional shapes, requires coordinated mechanical forces. Most current literature ascribes contractile forces derived from actomyosin networks as the major driver of tissue morphogenesis. Recent works from diverse species have shown that pressure derived from fluids can generate deformations necessary for tissue morphogenesis. In this review, we discuss how hydrostatic pressure is generated at the cellular and tissue level and how the pressure can cause deformations. We highlight and review findings demonstrating the mechanical roles of pressures from fluid-filled lumens and viscous gel-like components of the extracellular matrix. We also emphasise the interactions and mechanochemical feedbacks between extracellular pressures and tissue behaviour in driving tissue remodelling. Lastly, we offer perspectives on the open questions in the field that will further our understanding to uncover new principles of tissue organisation during development.
Keywords: Epithelial fluid transport; Epithelial lumen; Hyaluronan; Hydrostatic pressure; Mechanochemical feedback; Tissue morphogenesis.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest No conflict of interest. Conflict of interest The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

