Thapsigargin: key to new host-directed coronavirus antivirals?
- PMID: 35534355
- PMCID: PMC9013669
- DOI: 10.1016/j.tips.2022.04.004
Thapsigargin: key to new host-directed coronavirus antivirals?
Abstract
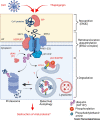
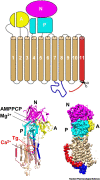
Despite the great success of vaccines that protect against RNA virus infections, and the development and clinical use of a limited number of RNA virus-specific drugs, there is still an urgent need for new classes of antiviral drugs against circulating or emerging RNA viruses. To date, it has proved difficult to efficiently suppress RNA virus replication by targeting host cell functions, and there are no approved drugs of this type. This opinion article discusses the recent discovery of a pronounced and sustained antiviral activity of the plant-derived natural compound thapsigargin against enveloped RNA viruses such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Middle East respiratory syndrome coronavirus (MERS-CoV), and influenza A virus. Based on its mechanisms of action, thapsigargin represents a new prototype of compounds with multimodal host-directed antiviral activity.
Keywords: COVID-19; ER membranes; ERAD; coronavirus; host-directed antivirals; proteome; thapsigargin.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

