Colossal barocaloric effects with ultralow hysteresis in two-dimensional metal-halide perovskites
- PMID: 35534457
- PMCID: PMC9085852
- DOI: 10.1038/s41467-022-29800-9
Colossal barocaloric effects with ultralow hysteresis in two-dimensional metal-halide perovskites
Abstract
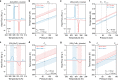
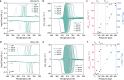
Pressure-induced thermal changes in solids-barocaloric effects-can be used to drive cooling cycles that offer a promising alternative to traditional vapor-compression technologies. Efficient barocaloric cooling requires materials that undergo reversible phase transitions with large entropy changes, high sensitivity to hydrostatic pressure, and minimal hysteresis, the combination of which has been challenging to achieve in existing barocaloric materials. Here, we report a new mechanism for achieving colossal barocaloric effects that leverages the large volume and conformational entropy changes of hydrocarbon order-disorder transitions within the organic bilayers of select two-dimensional metal-halide perovskites. Significantly, we show how the confined nature of these order-disorder phase transitions and the synthetic tunability of layered perovskites can be leveraged to reduce phase transition hysteresis through careful control over the inorganic-organic interface. The combination of ultralow hysteresis and high pressure sensitivity leads to colossal reversible isothermal entropy changes (>200 J kg-1 K-1) at record-low pressures (<300 bar).
© 2022. The Author(s).
Conflict of interest statement
J.S. and J.A.M. are inventors on a patent application related to this work held and submitted by Harvard University that covers barocaloric properties of two-dimensional perovskites and related compounds. The remaining authors declare no competing interests.
Figures







References
-
- Dupont, J. L., Domanski, P., Lebrun, P. & Ziegler, F. The Role of Refrigeration in the Global Economy. 38th Note on Refrigeration Technologies (International Institute of Refrigeration, 2019).
-
- Coulomb, D., Dupont, J.-L. & Morlet, V. The Impact of the Refrigeration Sector on Climate Change. 35th Note on Refrigeration Technologies (International Institute of Refrigeration, 2017).
-
- International Enegy Agency. The Future of Cooling: Opportunities for Energy-Efficient Air Conditioning (IEA, 2018).
-
- Office of Energy Efficiency and Renewable Energy. Energy Savings Potential and RD&D Opportunities for Commercial Building HVAC Systems (U.S. Department of Energy, 2017).
LinkOut - more resources
Full Text Sources

