Outpacing conventional nicotinamide hydrogenation catalysis by a strongly communicating heterodinuclear photocatalyst
- PMID: 35534473
- PMCID: PMC9085789
- DOI: 10.1038/s41467-022-30147-4
Outpacing conventional nicotinamide hydrogenation catalysis by a strongly communicating heterodinuclear photocatalyst
Abstract
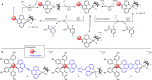
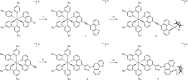
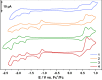
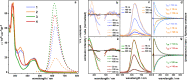
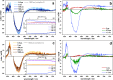
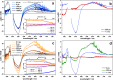
Unequivocal assignment of rate-limiting steps in supramolecular photocatalysts is of utmost importance to rationally optimize photocatalytic activity. By spectroscopic and catalytic analysis of a series of three structurally similar [(tbbpy)2Ru-BL-Rh(Cp*)Cl]3+ photocatalysts just differing in the central part (alkynyl, triazole or phenazine) of the bridging ligand (BL) we are able to derive design strategies for improved photocatalytic activity of this class of compounds (tbbpy = 4,4´-tert-butyl-2,2´-bipyridine, Cp* = pentamethylcyclopentadienyl). Most importantly, not the rate of the transfer of the first electron towards the RhIII center but rather the rate at which a two-fold reduced RhI species is generated can directly be correlated with the observed photocatalytic formation of NADH from NAD+. Interestingly, the complex which exhibits the fastest intramolecular electron transfer kinetics for the first electron is not the one that allows the fastest photocatalysis. With the photocatalytically most efficient alkynyl linked system, it is even possible to overcome the rate of thermal NADH formation by avoiding the rate-determining β-hydride elimination step. Moreover, for this photocatalyst loss of the alkynyl functionality under photocatalytic conditions is identified as an important deactivation pathway.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Tachibana Y, Vayssieres L, Durrant JR. Artificial photosynthesis for solar water-splitting. Nat. Photonics. 2012;6:511–518. doi: 10.1038/nphoton.2012.175. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

