Gut microbiota regulates acute myeloid leukaemia via alteration of intestinal barrier function mediated by butyrate
- PMID: 35534496
- PMCID: PMC9085760
- DOI: 10.1038/s41467-022-30240-8
Gut microbiota regulates acute myeloid leukaemia via alteration of intestinal barrier function mediated by butyrate
Abstract
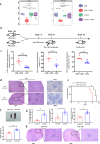
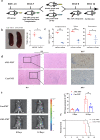
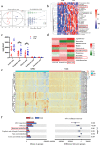
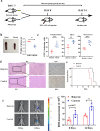
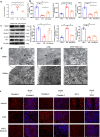
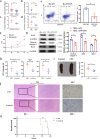
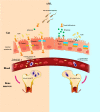
The gut microbiota has been linked to many cancers, yet its role in acute myeloid leukaemia (AML) progression remains unclear. Here, we show decreased diversity in the gut microbiota of AML patients or murine models. Gut microbiota dysbiosis induced by antibiotic treatment accelerates murine AML progression while faecal microbiota transplantation reverses this process. Butyrate produced by the gut microbiota (especially Faecalibacterium) significantly decreases in faeces of AML patients, while gavage with butyrate or Faecalibacterium postpones murine AML progression. Furthermore, we find the intestinal barrier is damaged in mice with AML, which accelerates lipopolysaccharide (LPS) leakage into the blood. The increased LPS exacerbates leukaemia progression in vitro and in vivo. Butyrate can repair intestinal barrier damage and inhibit LPS absorption in AML mice. Collectively, we demonstrate that the gut microbiota promotes AML progression in a metabolite-dependent manner and that targeting the gut microbiota might provide a therapeutic option for AML.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Gut microbiota promotes macrophage M1 polarization in hepatic sinusoidal obstruction syndrome via regulating intestinal barrier function mediated by butyrate.Gut Microbes. 2024 Jan-Dec;16(1):2377567. doi: 10.1080/19490976.2024.2377567. Epub 2024 Jul 16. Gut Microbes. 2024. PMID: 39012957 Free PMC article.
-
The metabolite butyrate produced by gut microbiota inhibits cachexia-associated skeletal muscle atrophy by regulating intestinal barrier function and macrophage polarization.Int Immunopharmacol. 2023 Nov;124(Pt B):111001. doi: 10.1016/j.intimp.2023.111001. Epub 2023 Oct 5. Int Immunopharmacol. 2023. PMID: 37804658
-
Gut microbiota is causally associated with poststroke cognitive impairment through lipopolysaccharide and butyrate.J Neuroinflammation. 2022 Apr 4;19(1):76. doi: 10.1186/s12974-022-02435-9. J Neuroinflammation. 2022. PMID: 35379265 Free PMC article.
-
Butyrate-producing bacteria and the gut-heart axis in atherosclerosis.Clin Chim Acta. 2020 Aug;507:236-241. doi: 10.1016/j.cca.2020.04.037. Epub 2020 May 4. Clin Chim Acta. 2020. PMID: 32376324 Review.
-
The gut microbiome in coronary artery disease and heart failure: Current knowledge and future directions.EBioMedicine. 2020 Feb;52:102649. doi: 10.1016/j.ebiom.2020.102649. Epub 2020 Feb 12. EBioMedicine. 2020. PMID: 32062353 Free PMC article. Review.
Cited by
-
The downregulation of tight junction proteins and pIgR in the colonic epithelium causes the susceptibility of EpCAM+/- mice to colitis and gut microbiota dysbiosis.Front Mol Biosci. 2024 Aug 12;11:1442611. doi: 10.3389/fmolb.2024.1442611. eCollection 2024. Front Mol Biosci. 2024. PMID: 39188786 Free PMC article.
-
Increased abundance of Firmicutes and depletion of Bacteroidota predicts poor outcome in chronic lymphocytic leukemia.Oncol Lett. 2024 Sep 17;28(5):552. doi: 10.3892/ol.2024.14685. eCollection 2024 Nov. Oncol Lett. 2024. PMID: 39328278 Free PMC article.
-
Advancements in the impact of human microbiota and probiotics on leukemia.Front Microbiol. 2024 Jul 3;15:1423838. doi: 10.3389/fmicb.2024.1423838. eCollection 2024. Front Microbiol. 2024. PMID: 39021626 Free PMC article. Review.
-
Gut microbiota plays pivotal roles in benign and malignant hematopoiesis.Blood Sci. 2024 Jul 17;6(4):e00200. doi: 10.1097/BS9.0000000000000200. eCollection 2024 Oct. Blood Sci. 2024. PMID: 39027904 Free PMC article. Review.
-
Microbiota Influences on Hematopoiesis and Blood Cancers: New Horizons?Blood Cancer Discov. 2023 Jul 5;4(4):267-275. doi: 10.1158/2643-3230.BCD-22-0172. Blood Cancer Discov. 2023. PMID: 37052501 Free PMC article. Review.
References
-
- Song X, et al. Incidence, survival, and risk factors for adults with acute myeloid leukemia not otherwise specified and acute myeloid leukemia with recurrent genetic abnormalities: analysis of the Surveillance, Epidemiology, and End Results (SEER) Database, 2001-2013. Acta Haematol. 2018;139:115–127. doi: 10.1159/000486228. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

