Arabidopsis nitrate-induced aspartate oxidase gene expression is necessary to maintain metabolic balance under nitrogen nutrient fluctuation
- PMID: 35534536
- PMCID: PMC9085827
- DOI: 10.1038/s42003-022-03399-5
Arabidopsis nitrate-induced aspartate oxidase gene expression is necessary to maintain metabolic balance under nitrogen nutrient fluctuation
Abstract
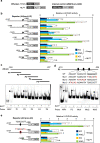
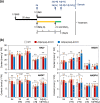
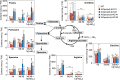
Nitrate is a nutrient signal that regulates growth and development through NLP transcription factors in plants. Here we identify the L-aspartate oxidase gene (AO) necessary for de novo NAD+ biosynthesis as an NLP target in Arabidopsis. We investigated the physiological significance of nitrate-induced AO expression by expressing AO under the control of the mutant AO promoter lacking the NLP-binding site in the ao mutant. Despite morphological changes and severe reductions in fresh weight, the loss of nitrate-induced AO expression resulted in minimum effects on NAD(H) and NADP(H) contents, suggesting compensation of decreased de novo NAD+ biosynthesis by reducing the growth rate. Furthermore, metabolite profiling and transcriptome analysis revealed that the loss of nitrate-induced AO expression causes pronounced impacts on contents of TCA cycle- and urea cycle-related metabolites, gene expression profile, and their modifications in response to changes in the nitrogen nutrient condition. These results suggest that proper maintenance of metabolic balance requires the coordinated regulation of multiple metabolic pathways by NLP-mediated nitrate signaling in plants.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Campbell WH. Nitrate reductase and its role in nitrate assimilation in plants. Physiol. Plant. 1988;74:214–219. doi: 10.1111/j.1399-3054.1988.tb04965.x. - DOI
-
- Scheible W-R, Lauerer M, Schulze E-D, Caboche M, Stitt M. Accumulation of nitrate in the shoot acts as a signal to regulate shoot-root allocation in tobacco. Plant J. 1997;11:671–691. doi: 10.1046/j.1365-313X.1997.11040671.x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

