Wnt/beta-catenin signaling confers ferroptosis resistance by targeting GPX4 in gastric cancer
- PMID: 35534546
- PMCID: PMC9613693
- DOI: 10.1038/s41418-022-01008-w
Wnt/beta-catenin signaling confers ferroptosis resistance by targeting GPX4 in gastric cancer
Abstract
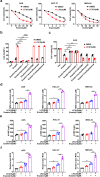
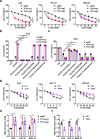
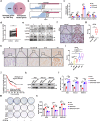
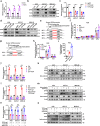
The development of chemotherapy resistance is the most vital obstacle to clinical efficacy in gastric cancer (GC). The dysregulation of the Wnt/beta-catenin signaling pathway is critically associated with GC development and chemotherapy resistance. Ferroptosis is a form of regulated cell death, induced by an iron-dependent accumulation of lipid peroxides during chemotherapy. However, whether the Wnt/beta-catenin signaling directly controls resistance to cell death, remains unclear. Here, we show that the activation of the Wnt/beta-catenin signaling attenuates cellular lipid ROS production and subsequently inhibits ferroptosis in GC cells. The beta-catenin/TCF4 transcription complex directly binds to the promoter region of GPX4 and induces its expression, resulting in the suppression of ferroptotic cell death. Concordantly, TCF4 deficiency promotes cisplatin-induced ferroptosis in vitro and in vivo. Thus, we demonstrate that the aberrant activation of the Wnt/beta-catenin signaling confers ferroptosis resistance and suggests a potential therapeutic strategy to enhance chemo-sensitivity for advanced GC patients.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Pan KF, Zhang L, Gerhard M, Ma JL, Liu WD, Ulm K, et al. A large randomised controlled intervention trial to prevent gastric cancer by eradication of Helicobacter pylori in Linqu County, China: baseline results and factors affecting the eradication. Gut. 2016;65:9–18. doi: 10.1136/gutjnl-2015-309197. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

