Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results
- PMID: 35534566
- PMCID: PMC9205778
- DOI: 10.1038/s41591-022-01800-8
Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results
Abstract
Despite success in hematologic malignancies, the treatment landscape of chimeric antigen receptor (CAR) T cell therapy for solid tumors remains limited. Claudin18.2 (CLDN18.2)-redirected CAR T cells showed promising efficacy against gastric cancer (GC) in a preclinical study. Here we report the interim analysis results of an ongoing, open-label, single-arm, phase 1 clinical trial of CLDN18.2-targeted CAR T cells (CT041) in patients with previously treated, CLDN18.2-positive digestive system cancers ( NCT03874897 ). The primary objective was safety after CT041 infusion; secondary objectives included CT041 efficacy, pharmacokinetics and immunogenicity. We treated 37 patients with one of three CT041 doses: 2.5 × 108, 3.75 × 108 or 5.0 × 108 cells. All patients experienced a grade 3 or higher hematologic toxicity. Grade 1 or 2 cytokine release syndrome (CRS) occurred in 94.6% of patients. No grade 3 or higher CRS or neurotoxicities, treatment-related deaths or dose-limiting toxicities were reported. The overall response rate (ORR) and disease control rate (DCR) reached 48.6% and 73.0%, respectively. The 6-month duration of response rate was 44.8%. In patients with GC, the ORR and DCR reached 57.1% and 75.0%, respectively, and the 6-month overall survival rate was 81.2%. These initial results suggest that CT041 has promising efficacy with an acceptable safety profile in patients with heavily pretreated, CLDN18.2-positive digestive system cancers, particularly in those with GC.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



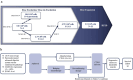
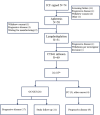
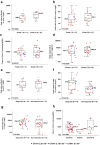
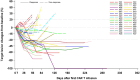

Comment in
-
CAR T cells show activity.Nat Rev Clin Oncol. 2022 Jul;19(7):427. doi: 10.1038/s41571-022-00648-8. Nat Rev Clin Oncol. 2022. PMID: 35606415 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

