Masking the immunotoxicity of interleukin-12 by fusing it with a domain of its receptor via a tumour-protease-cleavable linker
- PMID: 35534574
- PMCID: PMC11155269
- DOI: 10.1038/s41551-022-00888-0
Masking the immunotoxicity of interleukin-12 by fusing it with a domain of its receptor via a tumour-protease-cleavable linker
Abstract
Immune-checkpoint inhibitors have shown modest efficacy against immunologically 'cold' tumours. Interleukin-12 (IL-12)-a cytokine that promotes the recruitment of immune cells into tumours as well as immune cell activation, also in cold tumours-can cause severe immune-related adverse events in patients. Here, by exploiting the preferential overexpression of proteases in tumours, we show that fusing a domain of the IL-12 receptor to IL-12 via a linker cleavable by tumour-associated proteases largely restricts the pro-inflammatory effects of IL-12 to tumour sites. In mouse models of subcutaneous adenocarcinoma and orthotopic melanoma, masked IL-12 delivered intravenously did not cause systemic IL-12 signalling and eliminated systemic immune-related adverse events, led to potent therapeutic effects via the remodelling of the immune-suppressive microenvironment, and rendered cold tumours responsive to immune-checkpoint inhibition. We also show that masked IL-12 is activated in tumour lysates from patients. Protease-sensitive masking of potent yet toxic cytokines may facilitate their clinical translation.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
A.M., J.I., J.L.M., and J.A.H are inventors on a patent application filed by the University of Chicago covering the technology described in this work. They and M.A.S. hold equity in Arrow Immune, Inc., which is developing the technology, and J.A.H. is an officer of that company. The other authors declare no competing interests.
Figures


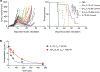

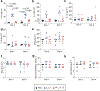
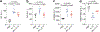






References
-
- Vitale I, Shema E, Loi S & Galluzzi L Intratumoral heterogeneity in cancer progression and response to immunotherapy. Nature medicine 27, 212–224 (2021). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

