Experimental validation of a voxel-based finite element model simulating femoroplasty of lytic lesions in the proximal femur
- PMID: 35534595
- PMCID: PMC9085891
- DOI: 10.1038/s41598-022-11667-x
Experimental validation of a voxel-based finite element model simulating femoroplasty of lytic lesions in the proximal femur
Abstract
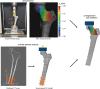
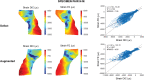
Femoroplasty is a procedure where bone cement is injected percutaneously into a weakened proximal femur. Uncertainty exists whether femoroplasty provides sufficient mechanical strengthening to prevent fractures in patients with femoral bone metastases. Finite element models are promising tools to evaluate the mechanical effectiveness of femoroplasty, but a thorough validation is required. This study validated a voxel-based finite element model against experimental data from eight pairs of human cadaver femurs with artificial metastatic lesions. One femur from each pair was left untreated, while the contralateral femur was augmented with bone cement. Finite element models accurately predicted the femoral strength in the defect (R2 = 0.96) and augmented (R2 = 0.93) femurs. The modelled surface strain distributions showed a good qualitative match with results from digital image correlation; yet, quantitatively, only moderate correlation coefficients were found for the defect (mean R2 = 0.78) and augmented (mean R2 = 0.76) femurs. This was attributed to the presence of vessel holes in the femurs and the jagged surface representation of our voxel-based models. Despite some inaccuracies in the surface measurements, the FE models accurately predicted the global bone strength and qualitative deformation behavior, both before and after femoroplasty. Hence, they can offer a useful biomechanical tool to assist clinicians in assessing the need for prophylactic augmentation in patients with metastatic bone disease, as well as in identifying suitable patients for femoroplasty.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Feng H, et al. CT-guided percutaneous femoroplasty (PFP) for the treatment of proximal femoral metastases. Pain Physician. 2016;19:E767–E773. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

