Optimization of DOTAP/chol Cationic Lipid Nanoparticles for mRNA, pDNA, and Oligonucleotide Delivery
- PMID: 35534697
- PMCID: PMC9084260
- DOI: 10.1208/s12249-022-02294-w
Optimization of DOTAP/chol Cationic Lipid Nanoparticles for mRNA, pDNA, and Oligonucleotide Delivery
Abstract
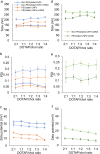
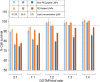
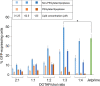
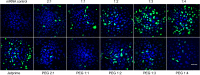
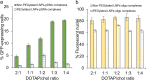
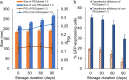
Lipid nanoparticles (LNPs) can be used as delivery vehicles for nucleic acid biotherapeutics. In fact, LNPs are currently being used in the Pfizer/BioNTech and Moderna COVID-19 vaccines. Cationic LNPs composed of 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP)/cholesterol (chol) LNPs have been classified as one of the most efficient gene delivery systems and are being tested in numerous clinical trials. The objective of this study was to examine the effect of the molar ratio of DOTAP/chol, PEGylation, and lipid to mRNA ratio on mRNA transfection, and explore the applications of DOTAP/chol LNPs in pDNA and oligonucleotide transfection. Here we showed that PEGylation significantly decreased mRNA transfection efficiency of DOTAP/chol LNPs. Among non-PEGylated LNP formulations, 1:3 molar ratio of DOTAP/chol in DOTAP/chol LNPs showed the highest mRNA transfection efficiency. Furthermore, the optimal ratio of DOTAP/chol LNPs to mRNA was tested to be 62.5 µM lipid to 1 μg mRNA. More importantly, these mRNA-loaded nanoparticles were stable for 60 days at 4 °C storage without showing reduction in transfection efficacy. We further found that DOTAP/chol LNPs were able to transfect pDNA and oligonucleotides, demonstrating the ability of these LNPs to transport the cargo into the cell nucleus. The influence of various factors in the formulation of DOTAP/chol cationic LNPs is thus described and will help improve drug delivery of nucleic acid-based vaccines and therapies.
Keywords: DOTAP/cholesterol; LNP-mRNA; lipid nanoparticles; mRNA delivery; transfection efficiency.
© 2022. The Author(s), under exclusive licence to American Association of Pharmaceutical Scientists.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Kawano T, Okuda T, Aoyagi H, Niidome T. Long circulation of intravenously administered plasmid DNA delivered with dendritic poly(L-lysine) in the blood flow. Journal of controlled release : official journal of the Controlled Release Society. 2004;99(2):329–337. doi: 10.1016/j.jconrel.2004.07.012. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

