Multiple myeloma with high-risk cytogenetics and its treatment approach
- PMID: 35534749
- PMCID: PMC9160142
- DOI: 10.1007/s12185-022-03353-5
Multiple myeloma with high-risk cytogenetics and its treatment approach
Abstract
Despite substantial advances in anti-myeloma treatments, early recurrence and death remain an issue in certain subpopulations. Cytogenetic abnormalities (CAs) are the most widely accepted predictors for poor prognosis in multiple myeloma (MM), such as t(4;14), t(14;16), t(14;20), gain/amp(1q21), del(1p), and del(17p). Co-existing high-risk CAs (HRCAs) tend to be associated with an even worse prognosis. Achievement of sustained minimal residual disease (MRD)-negativity has recently emerged as a surrogate for longer survival, regardless of cytogenetic risk. Information from newer clinical trials suggests that extended intensified treatment can help achieve MRD-negativity in patients with HRCAs, which may lead to improved outcomes. Therapy should be considered to include a 3- or 4-drug induction regimen (PI/IMiD/Dex or PI/IMiD/Dex/anti-CD38 antibody), auto-transplantation, and consolidation/maintenance with lenalidomide ± a PI. Results from ongoing clinical trials for enriched high-risk populations will reveal the precise efficacy of the investigated regimens. Genetic abnormalities of MM cells are intrinsic critical factors determining tumor characteristics, which reflect the natural course and drug sensitivity of the disease. This paper reviews the clinicopathological features of genomic abnormalities related to adverse prognosis, focusing on HRCAs that are the most relevant in clinical practice, and outline current optimal therapeutic approaches for newly diagnosed MM with HRCAs.
Keywords: Multiple myeloma; del(17p); del(1p); gain/amp(1q21); t(14;16).
© 2022. The Author(s).
Conflict of interest statement
The author received honoraria and/or membership on an entity's board of directors, speakers’ bureau, or advisory committees from Celgene, Janssen, Takeda, Ono, Bristol-Myers Squibb (BMS), Novartis, Daiichi Sankyo, Kyowa Kirin, Eisai, Nihon-Shinyaku, Pfizer, AbbVie, Otsuka, Shionogi, Mundi, CSL Behring, and Merck Sharp & Dohme. The author received research funding from BMS, Ono, Kyowa Kirin, Sanofi, Takeda, and Celgene.
Figures
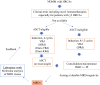
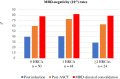
References
-
- Fernández de Larrea C, Kyle R, Rosiñol L, Paiva B, Engelhardt M, Usmani S, et al. Primary plasma cell leukemia: consensus definition by the International Myeloma Working Group according to peripheral blood plasma cell percentage. Blood Cancer J. 2021;11(12):192. doi: 10.1038/s41408-021-00587-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

