Natural product myricetin is a pan-KDM4 inhibitor which with poly lactic-co-glycolic acid formulation effectively targets castration-resistant prostate cancer
- PMID: 35534851
- PMCID: PMC9082844
- DOI: 10.1186/s12929-022-00812-3
Natural product myricetin is a pan-KDM4 inhibitor which with poly lactic-co-glycolic acid formulation effectively targets castration-resistant prostate cancer
Abstract
Background: Castration-resistant prostate cancer (CRPC) with sustained androgen receptor (AR) signaling remains a critical clinical challenge, despite androgen depletion therapy. The Jumonji C-containing histone lysine demethylase family 4 (KDM4) members, KDM4A‒KDM4C, serve as critical coactivators of AR to promote tumor growth in prostate cancer and are candidate therapeutic targets to overcome AR mutations/alterations-mediated resistance in CRPC.
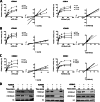
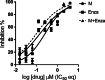
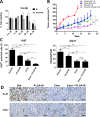
Methods: In this study, using a structure-based approach, we identified a natural product, myricetin, able to block the demethylation of histone 3 lysine 9 trimethylation by KDM4 members and evaluated its effects on CRPC. A structure-based screening was employed to search for a natural product that inhibited KDM4B. Inhibition kinetics of myricetin was determined. The cytotoxic effect of myricetin on various prostate cancer cells was evaluated. The combined effect of myricetin with enzalutamide, a second-generation AR inhibitor toward C4-2B, a CRPC cell line, was assessed. To improve bioavailability, myricetin encapsulated by poly lactic-co-glycolic acid (PLGA), the US food and drug administration (FDA)-approved material as drug carriers, was synthesized and its antitumor activity alone or with enzalutamide was evaluated using in vivo C4-2B xenografts.
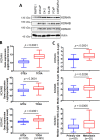
Results: Myricetin was identified as a potent α-ketoglutarate-type inhibitor that blocks the demethylation activity by KDM4s and significantly reduced the proliferation of both androgen-dependent (LNCaP) and androgen-independent CRPC (CWR22Rv1 and C4-2B). A synergistic cytotoxic effect toward C4-2B was detected for the combination of myricetin and enzalutamide. PLGA-myricetin, enzalutamide, and the combined treatment showed significantly greater antitumor activity than that of the control group in the C4-2B xenograft model. Tumor growth was significantly lower for the combination treatment than for enzalutamide or myricetin treatment alone.
Conclusions: These results suggest that myricetin is a pan-KDM4 inhibitor and exhibited potent cell cytotoxicity toward CRPC cells. Importantly, the combination of PLGA-encapsulated myricetin with enzalutamide is potentially effective for CRPC.
Keywords: Castration-resistant prostate cancer; Enzalutamide; Histone lysine demethylase family 4 (KDM4); Myricetin; Poly lactic-co-glycolic acid (PLGA).
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Targeting KDM4B that coactivates c-Myc-regulated metabolism to suppress tumor growth in castration-resistant prostate cancer.Theranostics. 2021 Jun 26;11(16):7779-7796. doi: 10.7150/thno.58729. eCollection 2021. Theranostics. 2021. PMID: 34335964 Free PMC article.
-
Discovery of a Novel Bifunctional Steroid Analog, YXG-158, as an Androgen Receptor Degrader and CYP17A1 Inhibitor for the Treatment of Enzalutamide-Resistant Prostate Cancer.J Med Chem. 2023 Jul 27;66(14):9972-9991. doi: 10.1021/acs.jmedchem.3c00880. Epub 2023 Jul 17. J Med Chem. 2023. PMID: 37458396
-
A novel androgen receptor antagonist JJ-450 inhibits enzalutamide-resistant mutant ARF876L nuclear import and function.Prostate. 2020 Mar;80(4):319-328. doi: 10.1002/pros.23945. Epub 2019 Dec 23. Prostate. 2020. PMID: 31868960 Free PMC article.
-
Androgen receptors in hormone-dependent and castration-resistant prostate cancer.Pharmacol Ther. 2013 Dec;140(3):223-38. doi: 10.1016/j.pharmthera.2013.07.003. Epub 2013 Jul 13. Pharmacol Ther. 2013. PMID: 23859952 Review.
-
Bipolar Androgen Therapy Followed by Androgen Receptor Inhibition as Sequential Therapy for Prostate Cancer.Oncologist. 2023 Jun 2;28(6):465-473. doi: 10.1093/oncolo/oyad055. Oncologist. 2023. PMID: 37027449 Free PMC article. Review.
Cited by
-
Targeting N-Methyl-lysine Histone Demethylase KDM4 in Cancer: Natural Products Inhibitors as a Driving Force for Epigenetic Drug Discovery.ChemMedChem. 2025 Feb 16;20(4):e202400682. doi: 10.1002/cmdc.202400682. Epub 2024 Nov 21. ChemMedChem. 2025. PMID: 39498961 Free PMC article. Review.
-
Recent studies on myricetin and its biological and pharmacological activities.EXCLI J. 2023 Nov 28;22:1223-1231. doi: 10.17179/excli2023-6571. eCollection 2023. EXCLI J. 2023. PMID: 38317860 Free PMC article. No abstract available.
-
Histone Demethylase Modulation: Epigenetic Strategy to Combat Cancer Progression.Epigenomes. 2023 May 17;7(2):10. doi: 10.3390/epigenomes7020010. Epigenomes. 2023. PMID: 37218871 Free PMC article. Review.
-
The role of KDM4A-mediated histone methylation on temozolomide resistance in glioma cells through the HUWE1/ROCK2 axis.Kaohsiung J Med Sci. 2024 Feb;40(2):161-174. doi: 10.1002/kjm2.12768. Epub 2023 Oct 24. Kaohsiung J Med Sci. 2024. PMID: 37873881 Free PMC article.
-
Myricetin: A Significant Emphasis on Its Anticancer Potential via the Modulation of Inflammation and Signal Transduction Pathways.Int J Mol Sci. 2023 Jun 2;24(11):9665. doi: 10.3390/ijms24119665. Int J Mol Sci. 2023. PMID: 37298616 Free PMC article. Review.
References
-
- Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Chen H, Kong X, Melamed J, Tepper CG, Kung HJ, Brodie AM, Edwards J, Qiu Y. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69(6):2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. - DOI - PMC - PubMed
-
- Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, Han M, Partin AW, Vessella RL, Isaacs WB, Bova GS, Luo J. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69(1):16–22. doi: 10.1158/0008-5472.CAN-08-2764. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 110-2320-B-007-006/Ministry of Science and Technology, Taiwan
- 105-2314-B-007-001/Ministry of Science and Technology, Taiwan
- 106-2314-B-007-001/Ministry of Science and Technology, Taiwan
- 107-2314-B-007-001/Ministry of Science and Technology, Taiwan
- 108-2320-B-007-001/Ministry of Science and Technology, Taiwan
- 108-2311-B-007-006-MY3/Ministry of Science and Technology, Taiwan
- 109-2327-B-007-001/Ministry of Science and Technology, Taiwan
- 109-2320-B-007-008/Ministry of Science and Technology, Taiwan
- 105-2314-B-400-019-MY3/Ministry of Science and Technology, Taiwan
- 105-2320-B-038-071-MY3/Ministry of Science and Technology, Taiwan
- 107-2320-B-038-055-MY3/Ministry of Science and Technology, Taiwan
- 108-2320-B-038-011-MY3/Ministry of Science and Technology, Taiwan
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

