Changes in glial cell phenotypes precede overt neurofibrillary tangle formation, correlate with markers of cortical cell damage, and predict cognitive status of individuals at Braak III-IV stages
- PMID: 35534858
- PMCID: PMC9082857
- DOI: 10.1186/s40478-022-01370-3
Changes in glial cell phenotypes precede overt neurofibrillary tangle formation, correlate with markers of cortical cell damage, and predict cognitive status of individuals at Braak III-IV stages
Abstract
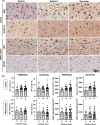
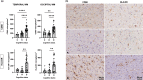
Clinico-pathological correlation studies show that some otherwise healthy elderly individuals who never developed cognitive impairment harbor a burden of Alzheimer's disease lesions (plaques and tangles) that would be expected to result in dementia. In the absence of comorbidities explaining such discrepancies, there is a need to identify other brain changes that meaningfully contribute to the cognitive status of an individual in the face of such burdens of plaques and tangles. Glial inflammatory responses, a universal phenomenon in symptomatic AD, show robust association with degree of cognitive impairment, but their significance in early tau pathology stages and contribution to the trajectory of cognitive decline at an individual level remain widely unexplored. We studied 55 brains from individuals at intermediate stages of tau tangle pathology (Braak III-IV) with diverging antemortem cognition (demented vs. non-demented, here termed `resilient'), and age-matched cognitively normal controls (Braak 0-II). We conducted quantitative assessments of amyloid and tau lesions, cellular vulnerability markers, and glial phenotypes in temporal pole (Braak III-IV region) and visual cortex (Braak V-VI region) using artificial-intelligence based semiautomated quantifications. We found distinct glial responses with increased proinflammatory and decreased homeostatic markers, both in regions with tau tangles (temporal pole) and without overt tau deposits (visual cortex) in demented but not in resilient. These changes were significantly associated with markers of cortical cell damage. Similar phenotypic glial changes were detected in the white matter of demented but not resilient and were associated with higher burden of overlying cortical cellular damage in regions with and without tangles. Our data suggest that changes in glial phenotypes in cortical and subcortical regions represent an early phenomenon that precedes overt tau deposition and likely contributes to cell damage and loss of brain function predicting the cognitive status of individuals at intermediate stages of tau aggregate burden (Braak III-IV).
Keywords: AD; Cognition; Cortical cell vulnerability; Glial phenotypes; Neurofibrillary tangles; White matter changes.
© 2022. The Author(s).
Figures







Similar articles
-
Dissecting phenotypic traits linked to human resilience to Alzheimer's pathology.Brain. 2013 Aug;136(Pt 8):2510-26. doi: 10.1093/brain/awt171. Epub 2013 Jul 3. Brain. 2013. PMID: 23824488 Free PMC article.
-
Tau Oligomer-Containing Synapse Elimination by Microglia and Astrocytes in Alzheimer Disease.JAMA Neurol. 2023 Nov 1;80(11):1209-1221. doi: 10.1001/jamaneurol.2023.3530. JAMA Neurol. 2023. PMID: 37812432 Free PMC article.
-
[F-18]-AV-1451 binding correlates with postmortem neurofibrillary tangle Braak staging.Acta Neuropathol. 2017 Oct;134(4):619-628. doi: 10.1007/s00401-017-1740-8. Epub 2017 Jun 13. Acta Neuropathol. 2017. PMID: 28612291 Free PMC article.
-
Neuropathology of Alzheimer's disease: a critical update.J Neural Transm Suppl. 1998;54:77-95. doi: 10.1007/978-3-7091-7508-8_8. J Neural Transm Suppl. 1998. PMID: 9850917 Review.
-
Lesions without symptoms: understanding resilience to Alzheimer disease neuropathological changes.Nat Rev Neurol. 2022 Jun;18(6):323-332. doi: 10.1038/s41582-022-00642-9. Epub 2022 Mar 24. Nat Rev Neurol. 2022. PMID: 35332316 Free PMC article. Review.
Cited by
-
Aqueous extractable nonfibrillar and sarkosyl extractable fibrillar Alzheimer's disease tau seeds have distinct properties.Acta Neuropathol Commun. 2024 Sep 9;12(1):145. doi: 10.1186/s40478-024-01849-1. Acta Neuropathol Commun. 2024. PMID: 39252090 Free PMC article.
-
Multidimensional MRI reveals cortical astrogliosis linked to dementia in Alzheimer's disease.Brain Commun. 2025 Jun 18;7(3):fcaf245. doi: 10.1093/braincomms/fcaf245. eCollection 2025. Brain Commun. 2025. PMID: 40585810 Free PMC article.
-
The relationships between neuroglial and neuronal changes in Alzheimer's disease, and the related controversies II: gliotherapies and multimodal therapy.J Cent Nerv Syst Dis. 2022 Nov 14;14:11795735221123896. doi: 10.1177/11795735221123896. eCollection 2022. J Cent Nerv Syst Dis. 2022. PMID: 36407561 Free PMC article. Review.
-
An aging, pathology burden, and glial senescence build-up hypothesis for late onset Alzheimer's disease.Nat Commun. 2023 Mar 25;14(1):1670. doi: 10.1038/s41467-023-37304-3. Nat Commun. 2023. PMID: 36966157 Free PMC article. Review.
-
Resilience to Alzheimer's disease associates with alterations in perineuronal nets.Alzheimers Dement. 2025 Feb;21(2):e14504. doi: 10.1002/alz.14504. Epub 2024 Dec 31. Alzheimers Dement. 2025. PMID: 39737731 Free PMC article.
References
-
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, Bi W, Cohen AD, Lopresti BJ, DeKosky ST, Halligan EM, Klunk WE. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. - DOI - PMC - PubMed
-
- Araque Caballero MÁ, Suárez-Calvet M, Duering M, Franzmeier N, Benzinger T, Fagan AM, Bateman RJ, Jack CR, Levin J, Dichgans M, Jucker M, Karch C, Masters CL, Morris JC, Weiner M, Rossor M, Fox NC, Lee J-H, Salloway S, Danek A, Goate A, Yakushev I, Hassenstab J, Schofield PR, Haass C, Ewers M. White matter diffusion alterations precede symptom onset in autosomal dominant Alzheimer’s disease. Brain. 2018;141:3065–3080. doi: 10.1093/brain/awy229. - DOI - PMC - PubMed
-
- Bachstetter AD, Van Eldik LJ, Schmitt FA, Neltner JH, Ighodaro ET, Webster SJ, Patel E, Abner EL, Kryscio RJ, Nelson PT. Disease-related microglia heterogeneity in the hippocampus of Alzheimer’s disease, dementia with Lewy bodies, and hippocampal sclerosis of aging. Acta Neuropathol Commun. 2015;3:32–32. doi: 10.1186/s40478-015-0209-z. - DOI - PMC - PubMed
-
- Barroeta-Espar I, Weinstock LD, Perez-Nievas BG, Meltzer AC, Siao Tick Chong M, Amaral AC, Murray ME, Moulder KL, Morris JC, Cairns NJ, Parisi JE, Lowe VJ, Petersen RC, Kofler J, Ikonomovic MD, López O, Klunk WE, Mayeux RP, Frosch MP, Wood LB, Gomez-Isla T. Distinct cytokine profiles in human brains resilient to Alzheimer’s pathology. Neurobiol Dis. 2019;121:327–337. doi: 10.1016/j.nbd.2018.10.009. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

