CaVβ-subunit dependence of forward and reverse trafficking of CaV1.2 calcium channels
- PMID: 35534894
- PMCID: PMC9082888
- DOI: 10.1186/s13041-022-00930-x
CaVβ-subunit dependence of forward and reverse trafficking of CaV1.2 calcium channels
Abstract
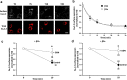
Auxiliary CaVβ subunits interact with the pore forming CaVα1 subunit to promote the plasma membrane expression of high voltage-activated calcium channels and to modulate the biophysical properties of Ca2+ currents. However, the effect of CaVβ subunits on channel trafficking to and from the plasma membrane is still controversial. Here, we have investigated the impact of CaVβ1b and CaVβ2a subunits on plasma membrane trafficking of CaV1.2 using a live-labeling strategy. We show that the CaVβ1b subunit is more potent in increasing CaV1.2 expression at the plasma membrane than the CaVβ2a subunit and that this effect is not related to modification of intracellular trafficking of the channel (i.e. neither forward trafficking, nor recycling, nor endocytosis). We conclude that the differential effect of CaVβ subunit subtypes on CaV1.2 surface expression is likely due to their differential ability to protect CaV1.2 from degradation.
Keywords: CaVβ auxiliary subunits; Calcium channel; Trafficking.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

