Quantification correction for free-breathing myocardial T1ρ mapping in mice using a recursively derived description of a T1ρ* relaxation pathway
- PMID: 35534901
- PMCID: PMC9082875
- DOI: 10.1186/s12968-022-00864-2
Quantification correction for free-breathing myocardial T1ρ mapping in mice using a recursively derived description of a T1ρ* relaxation pathway
Abstract
Background: Fast and accurate T1ρ mapping in myocardium is still a major challenge, particularly in small animal models. The complex sequence design owing to electrocardiogram and respiratory gating leads to quantification errors in in vivo experiments, due to variations of the T1ρ relaxation pathway. In this study, we present an improved quantification method for T1ρ using a newly derived formalism of a T1ρ* relaxation pathway.
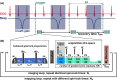
Methods: The new signal equation was derived by solving a recursion problem for spin-lock prepared fast gradient echo readouts. Based on Bloch simulations, we compared quantification errors using the common monoexponential model and our corrected model. The method was validated in phantom experiments and tested in vivo for myocardial T1ρ mapping in mice. Here, the impact of the breath dependent spin recovery time Trec on the quantification results was examined in detail.
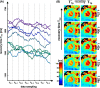
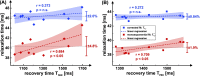
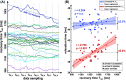
Results: Simulations indicate that a correction is necessary, since systematically underestimated values are measured under in vivo conditions. In the phantom study, the mean quantification error could be reduced from - 7.4% to - 0.97%. In vivo, a correlation of uncorrected T1ρ with the respiratory cycle was observed. Using the newly derived correction method, this correlation was significantly reduced from r = 0.708 (p < 0.001) to r = 0.204 and the standard deviation of left ventricular T1ρ values in different animals was reduced by at least 39%.
Conclusion: The suggested quantification formalism enables fast and precise myocardial T1ρ quantification for small animals during free breathing and can improve the comparability of study results. Our new technique offers a reasonable tool for assessing myocardial diseases, since pathologies that cause a change in heart or breathing rates do not lead to systematic misinterpretations. Besides, the derived signal equation can be used for sequence optimization or for subsequent correction of prior study results.
Keywords: Cardiac; Correction; Mapping; Quantitative MRI; Radial; Spin-lock; T1rho; T1ρ.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Fast myocardial T1ρ mapping in mice using k-space weighted image contrast and a Bloch simulation-optimized radial sampling pattern.MAGMA. 2022 Apr;35(2):325-340. doi: 10.1007/s10334-021-00951-y. Epub 2021 Sep 7. MAGMA. 2022. PMID: 34491466 Free PMC article.
-
Accelerated 3D free-breathing high-resolution myocardial T1ρ mapping at 3 Tesla.Magn Reson Med. 2022 Dec;88(6):2520-2531. doi: 10.1002/mrm.29417. Epub 2022 Aug 31. Magn Reson Med. 2022. PMID: 36054715
-
Respiratory motion-compensated high-resolution 3D whole-heart T1ρ mapping.J Cardiovasc Magn Reson. 2020 Feb 3;22(1):12. doi: 10.1186/s12968-020-0597-5. J Cardiovasc Magn Reson. 2020. PMID: 32014001 Free PMC article.
-
Free-breathing simultaneous native myocardial T1, T2 and T1ρ mapping with Cartesian acquisition and dictionary matching.J Cardiovasc Magn Reson. 2023 Nov 9;25(1):63. doi: 10.1186/s12968-023-00973-6. J Cardiovasc Magn Reson. 2023. PMID: 37946191 Free PMC article.
-
Errors in quantitative T1rho imaging and the correction methods.Quant Imaging Med Surg. 2015 Aug;5(4):583-91. doi: 10.3978/j.issn.2223-4292.2015.08.05. Quant Imaging Med Surg. 2015. PMID: 26435922 Free PMC article. Review.
Cited by
-
Robust cardiac mapping at 3T using adiabatic spin-lock preparations.Magn Reson Med. 2023 Oct;90(4):1363-1379. doi: 10.1002/mrm.29713. Epub 2023 May 28. Magn Reson Med. 2023. PMID: 37246420 Free PMC article.
References
-
- Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 2021;143(8):e254–e743. doi: 10.1161/CIR.0000000000000950. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

