Rifaximin Vs. Norfloxacin for Spontaneous Bacterial Peritonitis Prophylaxis: A Randomized Controlled Trial
- PMID: 35535057
- PMCID: PMC9077172
- DOI: 10.1016/j.jceh.2021.08.010
Rifaximin Vs. Norfloxacin for Spontaneous Bacterial Peritonitis Prophylaxis: A Randomized Controlled Trial
Abstract
Background: Spontaneous bacterial peritonitis (SBP) heralds increased mortality in cirrhosis, mandating strategies for prophylaxis. Norfloxacin has been the recommended choice for SBP prevention. However, its use has raised concerns about antibiotic resistance. Rifaximin has been suggested as an alternative. We investigated the efficacy of rifaximin against norfloxacin in primary and secondary prophylaxis of SBP.
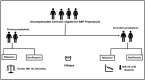
Methods: In this open-labeled randomized trial, patients with either advanced cirrhosis having ascitic fluid protein levels (<1.5 g/l), Child-Pugh score ≥9 points, serum bilirubin ≥3 mg/dl or impaired renal function (primary prophylaxis group), or those with prior SBP (secondary prophylaxis group) received either norfloxacin (400 mg once daily) or rifaximin (550 mg twice daily). All patients were followed for six months, with the primary endpoint being the development of incident SBP.
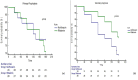
Results: 142 patients were assessed for eligibility, of which 132 met the enrolment criteria; 12 were lost to follow-up, while 4 discontinued treatment. In patients on primary prophylaxis, occurrence of SBP was similar (14.3% vs. 24.3%, P = 0.5), whereas in secondary prophylaxis SBP recurrence was lower with rifaximin (7% vs. 39% P = 0.004). Rifaximin significantly reduced the odds for SBP development in secondary prophylaxis [OR (95% CI0.14 (0.02-0.73; P = 0.02)]. Patients receiving rifaximin as secondary prophylaxis also had fewer episodes of hepatic encephalopathy (23.1% vs. 51.5%, P = 0.02). 180-day survival between the arms in either group was similar (P = 0.5, P = 0.2).
Conclusion: In comparison to norfloxacin, rifaximin significantly reduces incident events of SBP, as well as HE when used as a secondary prophylaxis, whereas for primary prophylaxis both have similar effects (NCT03695705).
Clinical trial registration: ClinicalTrials.gov number: NCT03695705.
Keywords: ACLF, Acute on chronic liver failure; AKI, Acute Kidney Injury; CONSORT, Consolidated Standards of Reporting Trials; CTP, Child-Turcotte-Pugh; HE, Hepatic encephalopathy; HRS, Hepatorenal syndrome; MELD, Model of end-stage liver disease; SAAG, Serum ascites albumin gradient; SBP; SBP, Spontaneous bacterial peritonitis; UGIB, Upper Gastrointestinal Bleed; VH, Variceal hemorrhage; antibiotic; ascites; cirrhosis; hepatic encephalopathy; infection; primary; resistance; secondary; survival.
© 2021 Indian National Association for Study of the Liver. Published by Elsevier B.V.
Figures
References
-
- Soni H., Kumar -M.P., Sharma V., et al. Antibiotics for prophylaxis of spontaneous bacterial peritonitis: systematic review & Bayesian network meta-analysis. Hepatol Int. 2020 Apr 7:1–5. - PubMed
-
- Yim H.J., Suh S.J., Jung Y.K., et al. Daily norfloxacin vs. weekly ciprofloxacin to prevent spontaneous bacterial peritonitis: a randomized controlled trial. Am J Gastroenterol. 2018 Aug 1;113:1167–1176. - PubMed
-
- European Association for the Study of the Liver EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018 Aug 1;69:406–460. - PubMed
-
- Goel A., Rahim U., Nguyen L.H., Stave C., Nguyen M.H. Systematic review with meta-analysis: rifaximin for the prophylaxis of spontaneous bacterial peritonitis. Aliment Pharmacol Ther. 2017 Dec;46:1029–1036. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

