Changes in Serum Interferon Gamma and Interleukin-10 in Relation to Direct-Acting Antiviral Therapy of Chronic Hepatitis C Genotype 4: A Pilot Study
- PMID: 35535108
- PMCID: PMC9077187
- DOI: 10.1016/j.jceh.2021.06.018
Changes in Serum Interferon Gamma and Interleukin-10 in Relation to Direct-Acting Antiviral Therapy of Chronic Hepatitis C Genotype 4: A Pilot Study
Abstract
Introduction: This study analyzes the changing levels of circulating inflammatory cytokines Interferon gamma (IFN-γ) and interleukin (IL)-10 (as the main cytokines of T-helper-1 and T-helper-2 immune responses) in patients with chronic hepatitis C virus (HCV) infection undergoing therapy with direct-acting antivirals (DAAs) and to correlate them with laboratory markers.
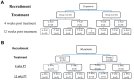
Methods: This Pilot study included 50 HCV monoinfected patients who received DAAs for 12 or 24 weeks. They were followed up monthly during therapy and 3 months after the end of the treatment. Liver disease was determined by transient elastography, in addition to FIB-4 indices. Analysis of IFN-gamma and IL-10 was carried out using an enzyme-linked immunosorbent assay.
Results: All patients carried HCV genotype 4. The Sustained virological response was 100% and 92% in cirrhotics and noncirrhotics, respectively. There was no significant difference between groups in baseline IL-10 or IFN-gamma. In noncirrhotics, IL-10 showed a significant reduction at Week 4 after treatment start. In cirrhotics, IL-10 showed a significant reduction at Week 4 after treatment starts and a significant reduction at Week 12 after the end of the treatment. At Week 12 after the end of the treatment, serum IL-10 levels were significantly lower in cirrhotics. IFN-γ showed nonsignificant changes in noncirrhotics. A significant increase of IFN-γ occurred in cirrhotics from Week 4 after treatment starts to 12 weeks after the end of the treatment. IFN-γ was significantly higher in cirrhotics at Week 12 after the end of the treatment. IFN-γ and IL-10 showed different correlations with laboratory markers.
Conclusion: Viral eradication induced by DAAs caused a significant change in IL-10 and IFN-gamma.
Keywords: ALT, alanine transaminase; AST, aspartate transaminase; CHC, chronic hepatitis c; DAA, Direct-acting antivirals; DAC, daclatasvir; DM, diabetes melliteus; EDTA, ethylenediaminetetraacetic acid; HCV, Hepatitis C virus; HTN, systemic hypertension; IFN-γ, interferon gamma; IL-10, interleukin 10; INR, international normalized ratio; NCCVH, National Committee for Control of Viral Hepatitis; SOF, sofosbuvir; STROBE, strengthening the reporting of observational studies in epidemiology; SVR, sustained virological response rates; Th, T-helper; cytokines; direct-acting antivirals; hepatitis C virus; interferon gamma; interleukin-10.
© 2021 Indian National Association for Study of the Liver. Published by Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Impact of different sofosbuvir based treatment regimens on the biochemical profile of chronic hepatitis C genotype 4 patients.Expert Rev Gastroenterol Hepatol. 2017 Aug;11(8):773-778. doi: 10.1080/17474124.2017.1326816. Epub 2017 May 15. Expert Rev Gastroenterol Hepatol. 2017. PMID: 28480808
-
Increased peripheral CD4+ regulatory T cells persist after successful direct-acting antiviral treatment of chronic hepatitis C.J Hepatol. 2017 May;66(5):888-896. doi: 10.1016/j.jhep.2016.12.019. Epub 2016 Dec 29. J Hepatol. 2017. PMID: 28040549 Albanian, English.
-
Restoring Inflammatory Mediator Balance after Sofosbuvir-Induced Viral Clearance in Patients with Chronic Hepatitis C.Mediators Inflamm. 2018 May 27;2018:8578051. doi: 10.1155/2018/8578051. eCollection 2018. Mediators Inflamm. 2018. PMID: 29977152 Free PMC article.
-
Treatment of hepatitis C virus genotype 3-infection.Liver Int. 2014 Feb;34 Suppl 1:18-23. doi: 10.1111/liv.12405. Liver Int. 2014. PMID: 24373074 Review.
-
Chronic Hepatitis C: Do Generics Work as Well as Branded Drugs?J Clin Exp Hepatol. 2017 Sep;7(3):253-261. doi: 10.1016/j.jceh.2017.08.003. Epub 2017 Sep 23. J Clin Exp Hepatol. 2017. PMID: 28970713 Free PMC article. Review.
Cited by
-
Serum omentin-1 is correlated with the severity of liver disease in patients with chronic hepatitis C.World J Hepatol. 2023 Dec 27;15(12):1315-1324. doi: 10.4254/wjh.v15.i12.1315. World J Hepatol. 2023. PMID: 38223417 Free PMC article.
References
-
- Cooke G.S., Lemoine M., Thursz M., et al. Viral hepatitis and the global burden of disease: a need to regroup. J Viral Hepat. 2013;20:600–601. - PubMed
-
- World Health Organization (WHO) World Health Organization; Geneva, Switzerland: 2017. Global Hepatitis Report 2017. Licence: CC BY-NC-SA 3.0 IGO.
-
- Lauer G.M., Walker B.D. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. - PubMed
LinkOut - more resources
Full Text Sources

