Evaluation of the Safety and Efficacy of Favipiravir in Adult Indian Patients with Mild-to-Moderate COVID-19 in a Real-World Setting
- PMID: 35535140
- PMCID: PMC9078344
- DOI: 10.2147/IJGM.S349241
Evaluation of the Safety and Efficacy of Favipiravir in Adult Indian Patients with Mild-to-Moderate COVID-19 in a Real-World Setting
Abstract
Purpose: To evaluate the safety and efficacy of favipiravir, which is prescribed for the treatment of patients with mild-to-moderate coronavirus disease 2019 (COVID-19) in India.
Patients and methods: This was a prospective, open-label, multicenter, single-arm postmarketing study conducted in India. Patients with mild-to-moderate COVID-19 received favipiravir (3600 mg [1800 mg orally twice daily] on the first day, followed by 800 mg orally twice daily, up to a maximum of 14 days) as a part of their treatment. The primary endpoints were to evaluate the safety of favipiravir by assessing the number of adverse events (AEs) and treatment-related AEs. The secondary endpoints were to evaluate the efficacy of favipiravir by assessing time to clinical cure, rate of clinical cure, time to pyrexia resolution, rate of oxygen requirement, and all-cause mortality.
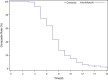
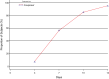
Results: A total of 1083 patients were enrolled in this study from December 2020 to June 2021. Adverse events were reported in 129 patients (11.9%), 116 (10.7%) of whom had mild AEs. Dose modification or withdrawal of favipiravir treatment was reported in four patients (0.37%). The median time to clinical cure and pyrexia resolution was 7 and 4 days, respectively. A total of 1036 patients (95.8%) exhibited clinical cure by day 14. Oxygen support was required by 15 patients (1.4%). One death was reported, which was unrelated to favipiravir.
Conclusion: In the real-world setting, favipiravir was well-tolerated, and no new safety signals were detected.
Keywords: COVID-19; SARS-CoV-2; antiviral; coronavirus; favipiravir.
© 2022 Reddy et al.
Conflict of interest statement
SP, SB, PD, AP, WW, SR, HB are full time employee of Glenmark Pharmaceuticals Ltd. PKR reports grants from Glenmark, during the conduct of the study. MK reports personal fees from Glenmark limited, during the conduct of the study. The authors report no other conflicts of interest in this work.
Figures
References
-
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available from: https://covid19.who.int/. Accessed October 25, 2021.
-
- WHO R&D Blueprint COVID 19 Experimental Treatments. COVID classification of treatment types; 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/covid-classificatio.... Accessed August 16, 2021.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous