Efficacy and Safety of Low-Dose Rituximab in Anti-MuSK Myasthenia Gravis Patients: A Retrospective Study
- PMID: 35535211
- PMCID: PMC9078430
- DOI: 10.2147/NDT.S358851
Efficacy and Safety of Low-Dose Rituximab in Anti-MuSK Myasthenia Gravis Patients: A Retrospective Study
Abstract
Purpose: To evaluate the efficacy and safety of low dosages of rituximab (RTX) in the treatment of MuSK-antibody-positive MG patients.
Patients and methods: We retrospectively analyzed the data of MuSK-antibody-positive MG patients who were treated with low dosages of RTX from January 2018 to October 2021. The long-term treatment response to RTX was assessed by Myasthenia Gravis Foundation of America (MGFA) post-interventional status (PIS), Myasthenia Gravis Status and Treatment Intensity (MGSTI), dosage of steroid, MG-related activities of daily living (MG-ADL) and myasthenic muscle score (MMS) at the end of follow-up.
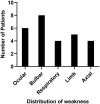
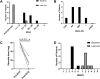
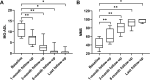
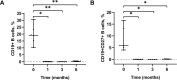
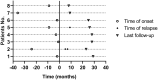
Results: Clinical improvement was observed in all eight patients with follow-up for 8 to 29 months after treatment. At the last visit, complete stable remission had been achieved in one patient, pharmacologic remission in three patients, minimal manifestations status in three patients and improved in one patient based on the MGFA-PIS criteria. MGSTI level 2 or better had been reached in six (75%) patients at the last visit. The steroid dosage decreased from 60 mg at baseline to 15 mg at the last follow-up (p = 0.011). The average MG-ADL score decreased from 11 (range 7 to 15) to 0 (range 0 to 3; p = 0.011), and the MMS improved from 38.5 (range 24 to 60) to 100 (range 90 to 100; p = 0.012). These differences were all statistically significant. During RTX treatment and subsequent follow-up, 1 patient reported minor post-infusion malaise.
Conclusion: Low-dose RTX is effective and safe for treating anti-MuSK antibody positive MG patients. A long-term response is observed after treatment. Larger prospective studies are required to provide further evidence.
Keywords: low-dose; muscle-specific kinase; myasthenia gravis; rituximab.
© 2022 Meng et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Efficacy and safety of rituximab in anti-MuSK myasthenia Gravis: a systematic review and meta-analysis.Sci Rep. 2025 Feb 28;15(1):7219. doi: 10.1038/s41598-025-90937-w. Sci Rep. 2025. PMID: 40021769 Free PMC article.
-
Clinical and laboratory remission with rituximab in anti-MuSK-positive myasthenia gravis.Ir J Med Sci. 2024 Dec;193(6):2989-2994. doi: 10.1007/s11845-024-03763-w. Epub 2024 Aug 1. Ir J Med Sci. 2024. PMID: 39088160 Free PMC article.
-
Promising efficacy of Low-Dose rituximab in Muscle specific kinase antibody positive Myasthenia Gravis.Neurosci Lett. 2024 Jan 1;818:137561. doi: 10.1016/j.neulet.2023.137561. Epub 2023 Nov 19. Neurosci Lett. 2024. PMID: 37984485
-
A retrospective study of the safety and efficacy of rituximab in Iranian patients with myasthenia gravis: A single-center experience.Curr J Neurol. 2022 Apr 4;21(2):91-97. doi: 10.18502/cjn.v21i2.10492. Curr J Neurol. 2022. PMID: 38011443 Free PMC article.
-
Efficacy and safety of low-dose rituximab in the treatment of myasthenia gravis: a systemic review and meta-analysis.Front Neurol. 2024 Sep 25;15:1439899. doi: 10.3389/fneur.2024.1439899. eCollection 2024. Front Neurol. 2024. PMID: 39385818 Free PMC article.
Cited by
-
Case report: Complex paraneoplastic syndromes in thymoma with nephrotic syndrome, cutaneous amyloidosis, myasthenia gravis, and Morvan's syndrome.Front Oncol. 2022 Nov 21;12:1002808. doi: 10.3389/fonc.2022.1002808. eCollection 2022. Front Oncol. 2022. PMID: 36479084 Free PMC article.
-
A Case Report of MuSK Antibody-Positive Myasthenia Gravis.Cureus. 2024 Jun 6;16(6):e61820. doi: 10.7759/cureus.61820. eCollection 2024 Jun. Cureus. 2024. PMID: 38975540 Free PMC article.
-
A pilot study of the immunological profile and efficacy of rituximab in muscle-specific kinase antibody-positive myasthenia gravis.Front Immunol. 2025 Jul 25;16:1624038. doi: 10.3389/fimmu.2025.1624038. eCollection 2025. Front Immunol. 2025. PMID: 40787458 Free PMC article.
-
Effect of low-dose rituximab treatment on autoimmune nodopathy with anti-contactin 1 antibody.Front Immunol. 2022 Jul 26;13:939062. doi: 10.3389/fimmu.2022.939062. eCollection 2022. Front Immunol. 2022. PMID: 35958552 Free PMC article.
-
Efficacy and safety of rituximab in anti-MuSK myasthenia Gravis: a systematic review and meta-analysis.Sci Rep. 2025 Feb 28;15(1):7219. doi: 10.1038/s41598-025-90937-w. Sci Rep. 2025. PMID: 40021769 Free PMC article.
References
LinkOut - more resources
Full Text Sources

